A Mad Hatter’s Tea Party? (with apologies to Lewis Carroll)
Posted on: Wednesday 26 January 2022
Author: Robert L Holland
This FPM Blog article has been prepared by Dr Robert L Holland.
It is provided for information and does not constitute advice or represent official FPM views or policy.
How to cite:
Holland, R L (2021), ‘A Mad Hatter’s Tea Party? (with apologies to Lewis Carroll): the weird and wonderful changing world of the regulation of devices and diagnostics. ’, FPM Blog, 13 January 2022. Available at: https://www.fpm.org.uk/blog/a-mad-hatters-tea-party-with-apologies-to-lewis-carroll/ (Accessed: <date>).
The weird and wonderful changing world of the regulation of devices and diagnostics.
When Alice came to the table, she naturally assumed that she would sit in one chair and enjoy her tea in the pleasant company of a collection of strange but interesting characters. Little did she know what would follow. Physicians working in the field of devices & diagnostics development, who are trying to understand the evolution of the relevant regulations in the EU and UK, may increasingly identify with Alice. It may feel as though they have fallen down a rabbit hole and entered a world where the rules keep changing, nothing is what it seems, and some people appear (like the Queen of Hearts) to be able to believe six impossible things before breakfast!
Devices and diagnostics regulation may seem an esoteric subject for pharmaceutical physicians, but as the bioscience world evolves, the deep connection between the two fields of pharmaceutical medicine and devices & diagnostics is becoming ever clearer. Pharmaceutical physicians will increasingly be required to incorporate knowledge of devices and diagnostics into their working practices. Recognising this, the Faculty of Pharmaceutical Medicine has convened the Medical Devices, Diagnostics and New Technologies Expert Group (MDDNTEG), which is considering including key aspects of devices & diagnostics development and regulation into the curriculum for training in Pharmaceutical Medicine. The expert group has recently responded to a public consultation exercise regarding the future of UK regulation of devices & diagnostics and the role that the MHRA will play in this regulation. This blog provides some background to the proposed changes and FPM’s response.

So, what is changing and what further changes are under discussion?
It is important to grasp that the starting point for devices and diagnostics regulation is quite different from that of medicines regulation and is much more akin to the kinds of rules applying to the manufacturers of consumer products. Thus, manufacturers must have a Quality Management System in place (typically ISO 13485 compliant) to guarantee that a product has been produced to the correct manufacturing standards. Manufacturers must show, through validation studies, that the device or diagnostic performs to the specifications claimed for the product and compile a technical file containing the evidence.
In Europe and much of the world, products typically have been brought to the market and entered clinical use with a Conformity Assessment “CE Mark” (or equivalent) through a process of self-certification. Oversight of this process and confirmation that the quality standards are being followed has not been delivered by Regulatory Authorities in these territories, but by third parties (many “for profit”) called Notified Bodies or Approved Bodies. The processes for diagnostic tests produced and utilised “in-house” by clinical laboratories complicates this regulatory landscape. Such laboratories are accredited by independent accreditation bodies (eg UKAS) – essentially, they are selling a service not a product.
Meanwhile, the landscape in the USA is different again: devices & diagnostics on the US market are directly regulated by the FDA through a separate group, the Center for Devices and Radiological Health (CDRH). However, the situation for clinical laboratories is more similar, with an accreditation process under the Clinical Laboratory Improvement Amendments (CLIA) regulation which requires certification by the Center for Medicare and Medicaid Services (CMS) often administered via the College of American Pathologists (CAP).
Self-certification seemed (and perhaps still seems) very appropriate for many of the products considered as medical devices (e.g. syringes and sterile gloves), and even for most diagnostic tests where the interpretation of the result of the test is always in the context of a plethora of additional information. However, as the medical world has changed, the potential risks to patients from devices and diagnostics have become more obvious1, and the interplay between devices, diagnostics and therapeutics can pose particular risks to patients. Obvious examples over the last decades include: drug eluting stents; inhaler devices; implantable devices (ranging from surgical meshes to defibrillators) and the emergence of “companion” diagnostics. Additionally, today there are entirely novel technologies emerging such as therapeutic apps, whole genome sequencing and pre-implantation genetic testing, where potential harms are far from fully understood.
The need for tighter control of the approval processes, and an emerging understanding of the importance of actual clinical data (safety in use, clinical validity and clinical utility) both pre-marketing and post-marketing (surveillance) has led to considerable rethinking of how devices and diagnostics should be regulated in Europe, and the new and much more stringent processes were to be implemented through EU legislation. The Regulations on Medical Devices (Regulation (EU) 2017/745) (in force as of 22 May 2021 after a year’s delay) and on In-Vitro Diagnostic Devices (Regulation (EU) 2017/746) (intended to be in force as of 26 May 2022, but delayed for perhaps 3-5 years depending upon the risk category of the IVD) changed the European legal framework for medical devices. They introduce new responsibilities for the EMA and for national competent authorities (e.g. the MHRA) in the assessment of certain categories of medical device and diagnostic that may place patients at a higher risk of harm.2
Whilst there is much to welcome in these changes from a medical practitioner’s perspective and perhaps from a public health perspective in terms of patient safety, some of the requirements seem to present almost insurmountable burdens for smaller device and diagnostics manufacturers. For example, it is thought that fewer than a quarter of the current CE-marked IVDs have an associated technical file containing all the data required under these new regulations.3 It’s relevant at this point to remember that most devices and diagnostics companies are generally not very profitable; they are small or medium enterprises producing relatively low-price products (often single-use per patient) and usually without the Intellectual Property normal for pharmaceuticals and thus very vulnerable to “workarounds” by competitors.
A further problem concerns the relative absence of a regulatory infrastructure. A review of the Dutch market found that, under the old regulations, only around 7% of IVDs would have required direct assessment by a Notified Body, whereas under the new regulations 84% would require this detailed level of scrutiny. Moreover, the new regulations require that the Notified Bodies themselves undergo a process of evaluation and accreditation to ensure that they have the skills, processes and resources to conduct a thorough review of a technical file and to audit a manufacturer; of the 22 EU Notified Bodies existing under the old regulations, only 4 had been recognised by the EMA by the end of 2020.4 This led to genuine fears amongst patients and their advocates that they might be deprived of novel devices and diagnostics (or even lose devices and diagnostics currently available), as was discussed eloquently and with great passion at FPM’s Annual Symposium in November 2020. Recognising that the IVD market was not ready for the required changes, despite the initial five-year grace period between the directive becoming law in 2017 and planned implementation date of 26 May 2022 and citing the slow pace of accreditation of the notified bodies, the EU Commission has recently (14 October 2021) agreed to defer full implementation for a further three to five years (depending on the risk category of the IVD).5
So where does this leave the UK?
Brexit has undoubtedly caused confusion but has opened opportunities for the UK. The Medicines and Medical Devices Act 20216 gives much greater flexibility for the UK to make its own decisions regarding healthcare product regulation. It allows for an extension of the remit of the MHRA towards a more direct role in the regulation of devices and diagnostics. The first and most direct example of this was the decision to replace CE-Marking for devices and diagnostics with a UK equivalent, the “UK CA”- mark, which was supposed to be required for products on the market 1 January 2021.7 On 24 August 2021, the MHRA announced that this requirement has been delayed by two years and on 1 January 2022 it was confirmed that EU CE-marking would still be recognised in the UK until mid-2023.7 Amongst these various announcements, two matters stand out: first, that the new UK-CA seems very similar in its scope, requirements for evidence and for audit to the EU MDR and EU IVDR (perhaps not surprisingly since the MHRA was intimately involved in the development of these directives); and second, that Northern Ireland sits in a unique position in the UK where products may still require CE-marking. As of 30 June 2021, only two UK organisations had been accredited as “UK Approved Bodies” and were able to grant UK Conformity Assessment for devices and diagnostics with a third organisation having a scope limited to Conformity Assessment of IVDs for the detection of trisomy 21. Since the UK CA mark is not recognised in the EU, the benefits to manufacturers in obtaining the “UK CA” do not seem obvious.
In the context of this rather unsatisfactory background, the MHRA has proposed a wide-ranging change in the way devices & diagnostics are regulated in the UK, including significant changes in the role of the MHRA itself. In a positive move, the purposes and intentions of these proposals have been explained in detail and a process of public consultation has been initiated8, to which the MDDNTEG, and colleagues from the Policy and Communications Group and other groups within FPM, has submitted a detailed response.
Broadly, we strongly supported many of the intentions of the proposals, which are aimed at reducing the risks of potential harm to patients by a more complete and thorough evaluation of devices & diagnostics before they are marketed and by more careful safety surveillance whilst on the market. In order to achieve this ambition, the MHRA is proposing changes to, and expansions of, its own role in these processes. In aggregate, the proposals appear to bring into regulation in the UK the outcomes intended by the EU MDR and IVDR (including a continuance of a risk-based classification system, with scrutiny and oversight commensurate with the level of risk that a particular device might bring).
However, the proposals seem to go further and be more all-encompassing. They include:
- an expansion of the scope of regulation to include products which may have no direct medical benefit but might still provide risks to patients (e.g., dermal fillers for cosmetic purposes)
- clarity that the regulations will apply to IT systems and algorithms (including AI and downloadable apps)
- detailed descriptions of how clinical validation and clinical utility studies must be conducted and regulated (basically, ensuring that ICH GCP is followed for all clinical performance evaluation studies)
- suggestions about a new role for the MHRA in the accreditation of clinical laboratories
- a proposal that the MHRA might, in certain circumstances, take on the role of an approved body and directly authorise new devices and diagnostics
- a lot of detailed and legalistic proposals for the oversight of Approved Bodies, manufacturers and their UK agents, and organisations which provide Unique Device Identifiers for registries for the purpose of safety surveillance.
Although FPM’s submission does very much support the broad thrust of these proposals, perhaps because they are so all-encompassing, a first reading might lead to the view that the UK could become a rather unwelcoming geography for the discovery, development and marketing of devices & Diagnostics. Some of the proposals seem to be rather ad-hoc (e.g. some of the suggestions regarding genetics tests, IVF, IT etc.) and don’t fit neatly into the broader thrust of the regulations. Others seem to recognise how burdensome much of these regulations might be to SMEs and academic institutes, suggesting that exemptions might be granted, or alternatives offered, including (perhaps self-defeating) alternative routes to the market (e.g. mutual recognition processes) and transitional arrangements. There is also a slight sense that some of the proposals are already a little out of date; for example, many genetics and genomics tests are readily available from providers ex-UK, and therapeutic and diagnostic apps invented ex-UK are easily downloaded onto smartphones. Sometimes as a nation, we tend to forget that we represent only 1% of the world population and that a large majority of life sciences R&D and manufacturing is conducted elsewhere.
MDDNTEG and FPM wish the MHRA well with their intentions to improve the public health (and the safety of participants in clinical trials) through these new proposed regulations and we hope our responses to several hundred probing questions, accompanied by detailed text commentary, will help in the evolution of regulations which will benefit health whilst supporting the UK’s life sciences industry.
At the Mad Hatter’s tea party, the time was always six o’clock and though Alice moved from chair to chair as she, the Mad Hatter and the March Hare moved places around the table, Alice never actually got any tea to eat or drink. We really hope the MHRA can make genuine progress with their intentions and that time does start to move forwards again!
Chair, Medical Devices, Diagnostics and New Technologies Expert Group of the Faculty of Pharmaceutical Medicine.

References
- www.immdsreview.org.uk/Report.html
- www.ema.europa.eu/en/human-regulatory/overview/medical-devices
- www.360dx.com/regulatory-news-fda-approvals/eu-proposes-phased-implementation-ivdr-observers-caution-challenges?
- www.team-nb.org/wp-content/uploads/2020/11/Team-NB-PositionPaper-Team-NB-consideration-paper-on-IVDR-Date-of-Application.pdf
- www.ec.europa.eu/commission/presscorner/detail/en/IP_21_5209
- www.bills.parliament.uk/bills/2700
- www.gov.uk/guidance/regulating-medical-devices-in-the-uk
- www.gov.uk/government/consultations/consultation-on-the-future-regulation-of-medical-devices-in-the-united-kingdom
More FPM blogs

The Future of Medicines and Innovation: A Systems Approach to Environmental Sustainability
Find out more

Celebrating National Mentoring Day: A Q&A with mentor Dr Felix Jackson FFPM
Celebrating national mentoring day

Greener Pharmaceutical Medicine; how we’re championing environmentally-focussed practices
Blog by our Chief Executive, Dr Marcia Philbin.

How the MHRA Will Involve Patients & Public at Every Step of the Regulatory Journey
Dr Liz Clark takes a look at the MHRA’s new Patient Involvement Strategy
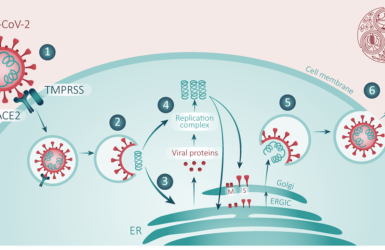
Inhalation therapies for COVID-19
Can we inhibit the initial stages of the infection of the respiratory tract?

Blog article: Preparedness Planning on Public Health Emergencies (PHEs)
The webinar series that has brought FPM into the heart of the COVID-19 pandemic.
COVID-19 – Characterisation of the paediatric disease and evolving therapeutic options
New blog article from Sankarasubramanian Rajaram, Sue Tansey et al
What you need to know about COVID-19 testing
An article by Robert L Holland FFPM and John Bagshaw of the British In Vitro Diagnostics Association

Principles for Clinical Studies into COVID-19
We have developed a number of principles for consideration by sponsors, investigators, ethics committees and journal editors.

COVID-19 – how pharmaceutical medicine is meeting the challenge
A report of the FPM survey of members on the effect of COVID-19 on their professional lives