Blog article: Preparedness Planning on Public Health Emergencies (PHEs)
Posted on: Wednesday 26 August 2020
Author: Dr Malcolm Brown and Francis P. Crawley FFPM (Hon)
This article has been prepared by Dr Malcolm Brown and Francis P. Crawley FFPM (Hon).
It is provided for information and does not constitute advice or represent official FPM views or policy.
How to cite:
Brown and Crawley (2020), ‘Preparedness Planning on Public Health Emergencies (PHEs)’, Faculty of Pharmaceutical Medicine blog, 26 August 2020. Available at: https://www.fpm.org.uk/blog/preparedness-planning-on-public-health-emergencies-phes/ (Accessed: <date>).
Introduction
As the FPM’s Ethics and Practice Committee’s webinar series on Preparedness Planning for Public Health Emergencies enters its final stages, some dominant themes have emerged. The journey started by reviewing the particular role of the pharmaceutical industry in responding to such emergencies (particularly in the context of COVID-19) and looked in turn at the impact of COVID-19 on clinical research in vaccines and therapeutics as well as the particular implications for paediatrics, rare diseases, and cancer. The final two webinars of the series cover data and knowledge sharing aspects and the emerging ethical issues.
The series has brought the Faculty into the heart of the COVID-19 pandemic, with regard to pharmaceutical medicine but also considering the global response. Inevitably attention has focused on COVID-19 and the public health emergency of the moment. Questions arose as to whether we had sufficiently learned the lessons of previous public health emergencies and if now, with a true pandemic running full course, we would make sufficient use of the webinars’ and other learnings in addressing future PHEs or even a second wave of the current one.
Opportunity Costs of COVID-19 Pandemic
The global impact of COVID-19, with its dramatic implications for research, medicine, healthcare, the economy, and even politics, has dominated discussions on health research and health-service resource allocation priorities worldwide for nearly all of 2020. We hear stories of people with serious health problems avoiding seeking treatment, either from fear of contracting the virus or out of an altruistic wish to avoid getting in the way of the ‘battle against COVID-19’. Cancer treatments are being deferred and life-saving surgery postponed. Live donor transplants are being delayed to protect both donor and recipient, though sometimes at a cost of the potential recipient becoming too ill to eventually receive the offered organ. Patients with orphan diseases are having to find new ways to receive their therapies while avoiding exposure to COVID-19. And many in low and middle-income countries (LMICs) have seen shortages in essential medicines, including HIV and tuberculous medicines, and healthcare access.
This webinar series allowed us to hear from the ‘front-line’, from experts from around the world, not just about the battle against the virus, but how other important aspects of healthcare delivery and research were being impacted. We investigated the major impact on clinical trials and why it was important that the cost of these delays in human terms should be recognised. Two areas of research in particular are worth calling out. For children with rare diseases, often their only hope of life-saving or life-changing treatment lies in being recruited into a clinical trial. This hope is removed when trials are paused or discontinued, in addition to delaying the time to market authorisation and broader access. For oncology research, suspending recruitment to a clinical trial means that those patients who might have benefited from the treatment becoming available, lost access to protocols and often will no longer be around when new improved treatment protocols based on clinical trials become available. These too are COVID-19 related deaths, albeit indirect. Both of these groups inevitably suffer from clinical trials that simply never occurred because resources were diverted to COVID-19 and the attention of the larger scientific and science-policy communities has been focused on the pandemic.
What the series has revealed is a general appreciation by patients and researchers of the systematic attention paid by pharmaceutical companies to addressing the needs of trials and their participants during COVID-19. Pharmaceutical companies have developed individual and coordinated responses to these issues. Whereas it may have seemed easier just to halt much of clinical research outside of COVID-19 due to overwhelming logistic problems, the approach has generally been a much more thoughtful response with each study considered individually down to the level of participating countries, participating centres, individual participants, and current numbers of COVID-infected individuals in the particular region. The risks of continuing have been weighed against opportunity costs to current and future patients of suspending.
How pharmaceutical companies, clinicians and researchers have adapted to fit changed circumstances
In the first webinar in the series, we considered the ways in which the pharmaceutical industry had contributed to the response to the COVID-19 pandemic. An immediate priority was ensuring the integrity of the supply-chain to make sure that medicines remained available for all patients and all conditions. This seems to have been successfully achieved, in many countries, despite major challenges in delivering anaesthetic drugs for the increased numbers of ventilated patients. However, as we learned further into the series, the challenges of maintaining an adequate supply of medicines to LMICs were not always successfully overcome.
The next priority was working with academia to develop vaccines, what appeared to be the only long-term route to minimising the impact of the SARS-COV-2 and hopefully seeing an end to infections. In a webinar specifically examining vaccine development, the major scientific obstacles were described, in addition to some of the societal and funding challenges.[1] The adaptations to the normal development timelines required to respond urgently to the crisis were described and the challenge of scaling-up manufacture were emphasised. Fortunately, the level of collaboration between all involved has been impressive.
In a search for effective treatments, many pharmaceutical companies have been taking a closer look at their existing portfolio of products and examining where their products’ known mode of action may indicate potential utility in directly attacking SARS-CoV-2 or in reducing the impact of an overly-aggressive immune response, which has often been the reason for the most severe impact on those infected. We were also given a word of caution regarding the consequences for those with the condition for which a product was indicated, if supplies should be diverted to treat those with COVID-19 where the outcomes might be much less certain.[2]
We also heard how changes have been made in the way studies are conducted, with visits to treatment centres reduced whenever possible. As many of us adjust to increased remote working and video-conferencing, patients involved in studies will also often welcome the possibility of at least some visits being virtual ones. Blood samples can be taken in the patient’s own homes by visiting phlebotomists. An interesting recent development in remote monitoring offers utility in the current COVID-19 impacted circumstances. The use of wearable devices is becoming commonplace and the Internet of Bodies (IoB) is starting to rival the Internet of Things (IoT) as the best way of exploiting technological advances to the benefit of us all.[3] It is likely that adaptation to the challenges of COVID-19 will bring lasting benefits to the way we conduct trials. Patients may be more willing to be recruited to studies if the burden of clinic visits is reduced and more monitoring can be done remotely.
Special groups
Treatment and clinical research in children present particular challenges in the face of a pandemic.[4] Supplies of medicines had mostly remained consistent, although in some particular countries (particularly in LMICs) this had been seriously affected with children missing out on routine vaccination.[5] Many studies had been halted to divert resources towards treating adult COVID-19 patients. The importance of effective international collaboration was emphasised by the panel, whether that be by global pharmaceutical companies, academic societies or organisations such as the World Health Organisation (WHO). A plea was also made for including children in vaccine trials once safety and efficacy had been established in studies in adults.
Another important vulnerable group considered was people living with rare diseases, many in the paediatric age group.[6] These patients were often advised to shelter at home so clinic visits became difficult, resulting in an increase in remote consultations. Maintaining safety in clinical trials and continuing these wherever possible was particularly important as access to potentially effective treatments was often only possible in the context of a clinical trial. Furthermore, for experimental treatments to eventually be available by prescription, it was important that research should continue. There was a danger that research might be halted due to an overwhelming focus on COVID-19.
The third special group considered was cancer research and treatment.[7] Here the opportunity costs of COVID-19 on cancer research have become well-known to the public due to recent statements of concern from prominent oncologists. This was very much the dominant theme in the FPM webinar on that topic with the importance of finding ways of continuing the research and finding innovative ways of doing so emphasised by all the panel members. We heard how some changes to the ways of conducting studies, such as fewer visits to hospitals and more home visits, introduced by necessity, may lead to more efficient approaches to clinical trial methodology continuing into the future.
Considerations for the future of pharmaceutical medicine in the context of COVID-19
Unfortunately, it does not look as if COVID-19 is going to disappear anytime soon. Accepting this likelihood is the first step. The next step is to make sure that we move on from clinical research being ‘all about COVID-19’ and the consequential opportunity costs to patients with other conditions. It may have been acceptable at one point in the pandemic to proceed in the belief that focussing everything on eradicating COVID-19, whilst potentially disadvantaging those with other conditions, was justified by considerations of the greater good. However, perhaps we have now moved beyond that point and COVID-19 will be around for a while yet, but research and healthcare should no longer be disproportionately focused on COVID-19. It is time for a more balanced view of what is important, and the FPM should continue to set an example of how to do the right thing for all patients.
From a new-found sense of fighting a ‘common enemy’, there are indeed some positives to be seen in the way that clinicians, academics and the pharmaceutical industry have more than ever seen the value of effective collaboration. The regulatory authorities and research ethics bodies have demonstrated their agility by streamlining review procedures whilst maintaining robust standards. Some light is emerging to overcome the darkness. The Ethics & Practice Committee will work to see that this new light finds a home in the Faculty’s education programmes and guidances.
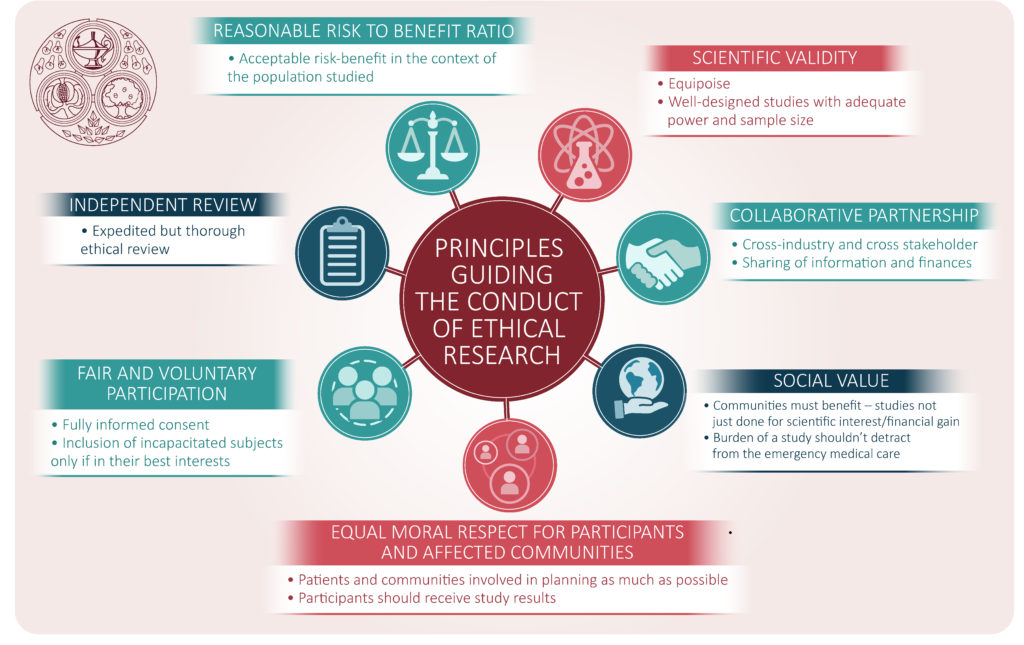
References
- Webinar 3 – Clinical Research and Development in PHEs (II): Vaccines, 1 July 2020
- Gurupira W. Webinar 2- Clinical Research and Development in PHEs – (I): Therapeutics, 17 June 2020
- Prichep E. Webinar 6 – Special Populations in PHEs (III): Cancer, 12 August 2020
- Webinar 4 – Special Populations in PHEs (I): Paediatrics, 15 July 2020
- Kruger M. Webinar 4 – Special Populations in PHEs (I): Paediatrics, 15 July 2020
- Webinar 5 – Special Populations in PHEs (II): Rare Diseases, 29 July 2020
- Webinar 6 – Special Populations in PHEs (III): Cancer, 12 August 2020
The Preparedness Planning on Public Health Emergencies webinar series
Webinar 1 - The Role of Pharmaceutical Medicine in Public Health Emergencies (PHEs)
Moderator:
Dr Alastair Benbow FFPM
Panellists:
Prof Tim Higenbottam PFPM
Prof Alan Boyd FFPM
Dr Penny Ward FFPM
Sponsor:
Indigo Medical
Webinar 2 - Clinical Research and Development in PHEs – (I): Therapeutics
Moderator:
Dr Penny Ward
Panellists:
Dr Susan Tansey FFPM
Mr Wilfred Gurupira
Dr Karol Szczukiewicz
Dr Jina Swartz
Webinar 3 - Clinical Research and Development in PHEs (II): Vaccines
Moderator:
Professor Alan Boyd FFPM
Panellists:
Dr. Jakob Cramer
Dr. Delon Human
Professor Jeffrey Almond
Professor David M. Salisbury
Webinar 4 - Special Populations in PHEs (I): Paediatrics
Moderator:
Professor Mark Turner FFPM (Hon)
Panellists:
Professor Barbara Bierer
Professor Mariana Kruger
Professor Sidnei Epelman
Webinar 5 - Special Populations in PHEs (II): Rare Diseases
Moderator:
Dr Zoya Panahloo FFPM
Panellists:
Tanya Collin-Histed
Professor Huma Arshad Cheema
Dr. Heather Lau
Emily Crossley
Webinar 6 - Webinar: Special Populations in PHEs (III): Cancer
Moderator:
Dr Cecilia Chisholm MFPM
Panellists:
Professor Igor Bondarenko
Dr Mariam Hassan
Dr Farah Rasheed
Professor Richard Sullivan
Dr Elissa Prichep

