The ethics of conducting clinical trials in the search for treatments and vaccines against COVID-19
Posted on: Tuesday 21 April 2020
Author: Dr Susan Tansey FFPM et al
This article has been prepared by Dr Susan Tansey FFPM, with input from Dr Ben Cottam, Dr Stuart Dollow FFPM, Dr Anthony Lockett, Dr Ian Mills FFPM and Dr Ivana Vranic FFPM.
It is provided for information and does not constitute advice or represent official FPM views or policy.
How to cite:
Tansey, S et al. (2020), ‘The Ethics of conducting clinical trials in the search for treatments and vaccines against COVID-19’, Faculty of Pharmaceutical Medicine blog, 21 April 2020. Available at: https://www.fpm.org.uk/blog/the-ethics-of-conducting-clinical-trials-in-the-search-for-treatments-and-vaccines-against-covid-19 (Accessed: <date>).
Introduction
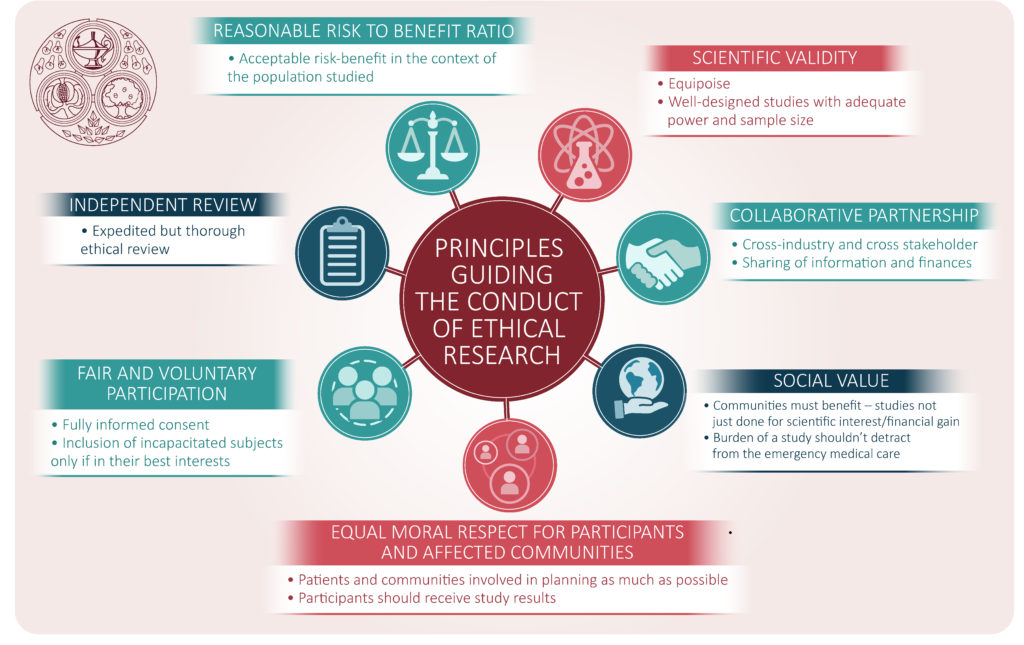
This article aims to address some of the ethical issues and dilemmas that may arise when planning and conducting clinical trials in the COVID-19 emergency. The World Health Organisation (WHO) addresses the emergency use of unproven interventions outside clinical research in its Guidance for managing ethical issues in infectious disease outbreaks1. The situations where it might be appropriate to consider provision of an experimental intervention outside clinical trials are listed and include when it is not possible to initiate clinical studies immediately. Therefore, where the possibility to participate in a clinical trial is available then this should be offered in preference to compassionate use provision of an unproven intervention. In addition, when experimental medicines are prescribed outside a clinical trial it is expected that the same ethical standards apply including the need for informed consent, ethical approval to be sought and that the intervention is monitored and results shared with the wider medical and scientific community. As discussed herein, clinical trials in an emergency should be planned and conducted to the same scientific rigour and ethical guidelines as would usually be expected. It would be unethical to include a patient in a clinical trial that will not generate robust evidence to support or refute the safety and efficacy of the investigational medicine being tested.
Therefore, we cover some of the important ethical issues when planning and conducting clinical trials in the COVID-19 pandemic, bearing in mind the ethical standards set out by the WHO in their guidance document on ethical standards to apply during a public health emergency distilled for use during the COVID-19 pandemic2.
Collaboration and expedited ethics approval
Multiple small trials are unlikely to generate the evidence needed, so it is important that global collaboration between clinicians, academics and across industry (between various companies) to plan well designed multi-centre international trials is promoted. These large global trials should be well designed, conducted to high ethical standards and ideally have a core protocol. The importance of having core protocols in emergency situations was emphasised by the experience with the PREVAIL II trial during the West Africa Ebola emergency in 2014-20163. In this case the trial of a triple monoclonal antibody cocktail ZMapp was terminated early due to the epidemic ending before completion of the study. This led to publication of inconclusive results which subsequently has led to future trials of potential Ebola therapies such as remdesivir being compared to the unproven ZMapp treatment instead of placebo. A core protocol where several potential treatments are evaluated simultaneously allows for the trial to be extended over several infectious disease outbreaks. The protocol should specify that efficacy data from a trial where recruitment is incomplete must not be released.
Examples of the large clinical trials with a core protocol that have been set up to investigate several therapeutic interventions for COVID-19 are the RECOVERY trial4 which has recently commenced in the UK and the WHO’s SOLIDARITY trial5. Both trials randomise patients with COVID-19 infection into 4 active treatment arms vs a no treatment arm.
Smaller well organised and conducted clinical trials also have a place where they will add to the evidence for a specific country or sub-population. It is just as important that these smaller trials are designed well.
In an emergency, there is a need to speed up the regulatory and ethical approval for trials. The UNESCO statement on COVID-196 emphasises that although the need for accelerated review and approval may be required, ethical principles must not be transgressed but adjusted to the exceptional circumstances to avoid any unnecessary delays. One area that ethics committees need to address is to ensure that protocols have accommodated the need for informed consent while bearing in mind that in the case of many of these studies, some of the potential participants will be very sick, confused or unconscious. In this situation either deferred consent or consent by proxy from a legal guardian and/or relative should be considered to ensure these very sick patients are able to participate in clinical trials.
Regulatory authorities including the MHRA have put in place arrangements for accelerated approval of clinical trials in the COVID-19 emergency. The UK’s Health Research Authority webpage related to COVID-19 research emphasises the importance of getting Research Ethics Committee review before starting research and the need for transparency.
Compassionate use vs clinical trials
As discussed earlier, where clinical trials are available, patients should be given the opportunity to participate in preference to being offered an unproven experimental treatment on a compassionate use basis. The issue of compassionate use is particularly relevant since many of the medicines being tested as potential therapies in COVID-19 infection are repurposed drugs and therefore are readily available to prescribe ‘off-label’. These investigational medicines include the malaria drugs chloroquine, hydroxychloroquine and the anti-viral remdesivir which have been used on a compassionate use basis for COVID-19 in several countries. Currently the evidence for the efficacy of these medicines in this indication is promising but not conclusive and there are risks e.g cardiac arrhythmias and deaths seen with the antimalarial drugs7. Remdesivir use in a small compassionate use protocol was reported, in which this antiviral was given to 61 subjects hospitalised with severe COVID-198. The report illustrates the importance of publishing this data as it is helpful in generating a hypothesis to be confirmed in a larger randomised controlled trial. Although the results contribute to the safety data in this indication, there can be no conclusions regarding efficacy since this was not designed as a randomised controlled study. Widespread compassionate use may undermine the ability to perform a controlled trial if unproven treatments become accepted as standard of care, and should be avoided. The large clinical trials discussed earlier will give us the best chance of finding out whether chloroquine, hydroxychloroquine, remdesivir and the other treatments to be tested are truly effective against COVID 19. As the medicines being investigated are all repurposed drugs, the pre-clinical and early clinical work has already been conducted for other indications and may not need to be repeated. Therefore, in countries where recruitment into a well designed clinical trial is possible this is likely to be preferable from an ethical standpoint.
On the other hand, in low income countries where the medical resources (such as ventilators and intensive care beds) to deal with the complications of COVID 19 infection are scarce or non-existent it could be argued that it might be ethical to provide hydroxychloroquine, a relatively cheaply available medicine, on a compassionate use basis to try and reduce the number of severe cases. However, it would be important to consider the risk-benefit taking into account the potential risks mentioned earlier.
Clinical trials in COVID-19 where there is no established effective treatment should be conducted against a placebo comparator or no treatment on top of standard of care. One of the advantages of these large multi-arm studies is that there is one placebo/no treatment arm against which all treatment arms will be tested, meaning that more patients get the chance of receiving a potentially active treatment than would be the case if there were several separate smaller trials each incorporating a placebo/no treatment arm. The designs are similar which will make it possible to compare patients across groups, possible in a meta-analysis . Additionally these trials are designed to be adaptive so that as soon as it becomes clear that a particular treatment is not effective that arm of the trial can be stopped for futility, therefore minimising the number of subjects to be recruited and shortening the time to get results.
Once a therapeutic treatment has been proven to have efficacy it is important that it is made available to patients where there is considered to be an acceptable benefit-risk ratio , so at this point it might be reasonable to consider compassionate use treatment until an investigational medicine that awaits approval in COVID-19 treatment is licensed. It is important to monitor and collect information on outcome and report results when a treatment is given in this setting, as was done in the remdesivir compassionate use protocol.
A prophylactic vaccine
Due to the need to expedite development of a prophylactic vaccine, the WHO has discussed that in some situations it might be acceptable to skip some steps in the pre-clinical development that would normally be expected9. This will in some cases mean that animal work can take place in parallel to the first-in-man studies and may require sharing data between researchers on animal models in other cases. The WHO commented that in cases where there is prior experience with a vaccine platform, it might not be necessary to repeat all pre-clinical work. It was acknowledged that with up to 40 vaccines in development, a practical issue is that there are simply not enough non- human primates (NHP) available to carry out the pre-clinical studies (that would usually be conducted) in all cases.
Even if it is considered ethical in some cases to speed up the process of vaccine development as described, it is extremely important that this is mentioned to potential trial participants during the consent process, clearly explaining the associated risks. In early clinical trials of a prophylactic vaccine it is generally healthy volunteers aged 18-55 who would be asked to participate. In order to give a fully informed consent, they also need to understand that they would not necessarily be protected against future coronavirus infection – at this stage there would be no evidence that an investigational vaccine was protective and in most cases the comparator would be a placebo vaccine. The reason for placebo control is that there is no effective vaccine against infection available to use as a comparator. Since COVID-19 infection is less likely to result in severe disease or death in these healthy volunteer subjects than in the elderly or those with an underlying disease, it is likely to be deemed ethical to use a placebo control. However, an alternative design could be considered such as the interesting ring vaccination trial design used in the Ebola ҫa suffit clinical trial10. Here, subjects in the ring surrounding the index case were randomised as a cluster to receive either immediate vs delayed vaccination. In this situation where subjects were at high risk of infection with Ebola it was not considered ethical to have a placebo comparator arm.
Although as mentioned it is usually most appropriate to conduct early vaccine clinical trials in healthy volunteers, if the eventual target population is to be a specific vulnerable population e.g. the elderly and/or pregnant women, then it would be ethical to consider including this population in later stage trials once there is early evidence of safety and tolerability and ideally an indication of protection against infection.
In a similar way to the collaboration we see in therapeutic clinical trials, the organization the Coalition for Epidemic Preparedness Innovation CEPI11 has been coordinating and supporting the development of several potential prophylactic vaccines by both academic groups and industry. Since at this early stage it is not possible to foresee which vaccine(s) might be successful, they are collaborating on several potential vaccines, in the hope of finding at least one that is safe and effective.
Conclusion
In conclusion, it is essential that despite the emergency of the COVID 19 pandemic that the global clinical research community makes every effort to plan and conduct well designed clinical trials to high ethical standards. It is important that inclusion and exclusion criteria are well defined and observed and patients are fully informed when consenting in this emergency situation. There should be expedited but thorough ethical and regulatory review of all clinical study applications ensuring there is an appropriate study design, comparator arm(s), clear eligibility criteria and adequate sample size. This will require cooperation and collaboration across many organisations and stakeholders including clinicians, academics, scientists, regulators and industry. The coalition proposed in the recent article by the COVID-19 Therapeutics Accelerator may assist in collaboration across organisations12.
In the emergency of the COVID-19 pandemic it is imperative that:
- Well designed clinical trials are planned and conducted to high ethical standard
- Study participants are fully informed of the benefits and risks
- Expedited but thorough ethical and regulatory review of all clinical research is conducted
- There is collaboration globally and across organizations involved in clinical trials
- Guidance for managing ethical issues in infectious disease outbreaks. 1.Disease Outbreaks. 2.Communicable Diseases. 3.Ethics. I. World Health Organization 2016
- World Health Organization (21 March 2020) Ethical standards for research during public health emergencies: distilling existing guidance to support COVID-19 R&D
- Creating a Framework for Conducting Randomized Clinical Trials during Disease Outbreaks NEJM 382;14 April 2, 2020
- https://www.recoverytrial.net/
- https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments
- UNESCO STATEMENT ON COVID-19: ETHICAL CONSIDERATIONS FROM A GLOBAL PERSPECTIVE
- https://www.ema.europa.eu/en/news/covid-19-chloroquine-hydroxychloroquine-only-be-used-clinical-trials-emergency-use-programmes
- Compassionate use of Remdesivir for Patients with severe Covid-19. NEJM 10.1056 April 10 2020.
- https://www.fpm.org.uk/blog/covid-19-vaccine-and-antiviral-drug-development/
- The ring vaccination trial: a novel cluster randomised controlled trial design to evaluate vaccine efficacy and effectiveness during outbreaks, with special reference to Ebola. Ebola ça suffit ring vaccination trial consortium. BMJ 2015; 351:3740
- Developing Covid-19 Vaccines at Pandemic Speed Lurie N et al. NEJM DOI: 10.1056 March 30, 2020.
- Global coalition to accelerate COVID-19 clinical research in resource-limited settings. The Lancet. Published online April 2 2020. The COVID-19 Clinical Research Coalition.