COVID-19 – Characterisation of the paediatric disease and evolving therapeutic options
Posted on: Friday 21 August 2020
Author: Sankarasubramanian Rajaram, Sue Tansey et al
This article has been prepared by Sankarasubramanian Rajaram, Sue Tansey, Peter Hession, Naila Aslam, Lisa Husband, Katharine Cheng, Allyah Abbas-Hanif.
It is provided for information and does not constitute advice or represent official FPM views or policy.
How to cite:
Rajaram, S et al. (2020), ‘COVID-19 – Characterisation of the paediatric disease and evolving therapeutic options’, Faculty of Pharmaceutical Medicine blog, 21 August 2020. Available at: https://www.fpm.org.uk/blog/covid-19-characterisation-of-the-paediatric-disease-and-evolving-therapeutic-options/ (Accessed: <date>).
Introduction
Severe illness with coronavirus disease 2019 (COVID-19), while less frequent in children than adults, is still significant, and with distinct clinical manifestations now being seen in sub-sets. Clinical research and the advancement of therapeutic developments means that this is a rapidly evolving area. This article, prepared by members of the FPM Paediatrics and Other Vulnerable Populations Expert Group, examines the characteristics of the disease in children, its social impact, and the treatment and vaccine options under development for use in this population.
Disease burden
COVID-19, due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), presents far less often in children (including adolescents) than in adults, accounting for fewer than 2% of all recorded cases[1-4]. Serology studies in Europe suggest that children are less likely than adults to get infected with SARS-CoV-2, although the magnitude of the difference is unclear[5,6]. Infection in children is more often asymptomatic than in adults, as suggested by evidence from secondary infection rates in households in China[7], although the proportion of asymptomatic infections remains unclear due to the wide variety of settings in which testing has been undertaken. The role of children in transmitting the virus is debated, although there is evidence that their role is limited[8,9]. Furthermore, although children present in a broadly similar manner to adults, they have less severe disease, including rates of admission to intensive care units that are under half of those seen in adults1. The case fatality mortality rate in children is also much lower than in adults, at under 1%[10], compared with a rate rising to up to 5% in 60 year old adults and reaching around 20% in those over 80 years old[11].
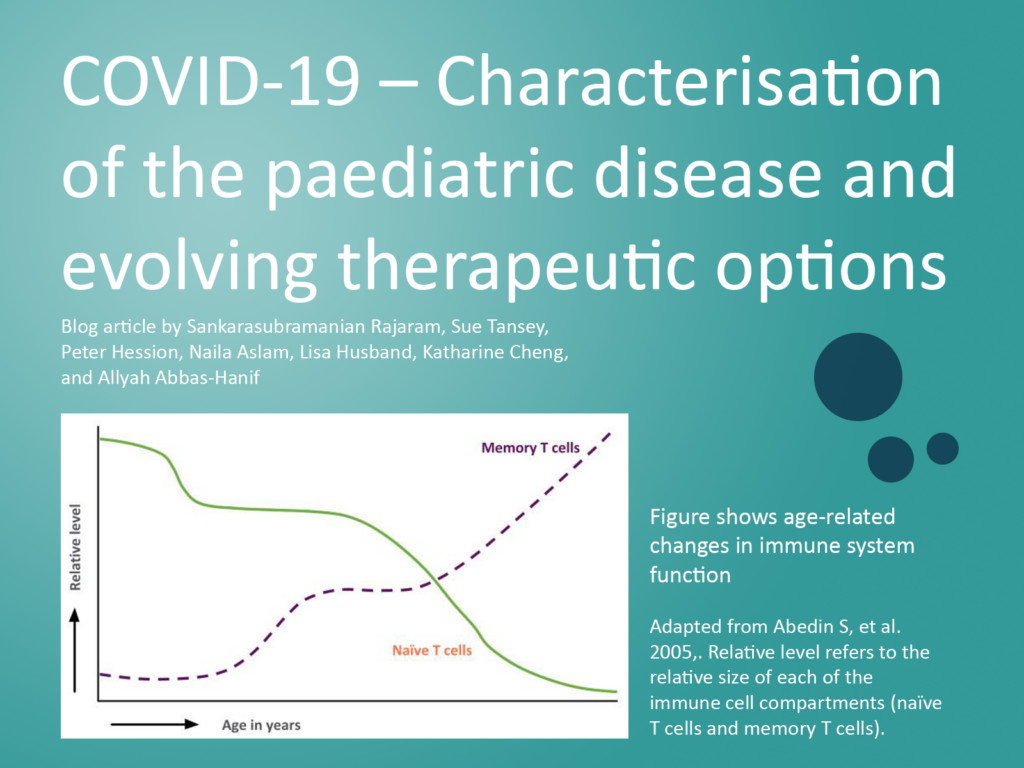
The reasons for the reduced infection rate and/or reduced severity of COVID-19 in children compared to adults are under investigation. A number of factors have been suggested that may contribute to this finding[12]. Children are often infected by an adult, in which case the infection would be by a second or third generation virus that could have reduced pathogenicity, as has been observed in previous coronavirus epidemics. Children have a lower prevalence than adults of co-morbidities associated with risk, such as hypertension and diabetes, and they may also have a greater pre-existing immunity due to more recent infection with related coronaviruses. Furthermore, angiotensin converting enzyme 2 (ACE-2), a cell surface protein and the main receptor by which SARS-CoV-2 enters cells, may be present at lower frequency in children than adults. Finally, children and adults have differences in their immune system, with children having a better innate response, and mucosal colonisation by viruses and bacteria is greater in children, which could limit the opportunities for colonisation and growth of SARS-CoV-2.
A generalised inflammatory syndrome in paediatric patients
Although most children with COVID-19 are asymptomatic or present with mild symptoms, over the last few months paediatricians have seen hospitalizations of children with a syndrome of fever and multisystem inflammation with some features similar to Kawasaki Disease temporally associated with COVID epidemics in Europe and the USA[13].
The exact aetiology of this syndrome – termed Paediatric Inflammatory multisystem syndrome (PIMS-TS) in the EU and Multisystem Inflammatary Syndrome of children (MIS-C) in the USA – is unknown. A range of presenting symptoms and signs and disease severity have been observed. No definitive diagnostic test is available. In some cases the condition presents similarly to Kawasaki disease, in which an infection with a common pathogen leads to an immune-mediated vasculitic response, with classic features including: non-purulent conjunctivitis, polymorphic rash, mucosal changes and swollen extremities; other cases have presented with a generalised hyper-inflammatory syndrome including some features of Kawasaki disease[13,14].
Of 58 children reported in England, most of whom tested positive for SARS-CoV-2 infection (26%) or had positive serology (87%), half required admission to a paediatric intensive care unit; coronary artery aneurysms and myocarditis were seen in some cases[14]. A similarly high proportion of serology-positive cases was observed in a French study of 21 children and adolescents[15]. These findings suggest the development of PIMS-TS may be the result of a post-viral immunological reaction. Of note, the majority of affected children – 69% in the English case series and 57% of children in the French case – were of black or Asian ancestry.
Most cases of PIMS-TS have been treated with high dose corticosteroids and intravenous immunoglobulins, with up to 81% of those admitted to paediatric intensive care requiring inotropic support due to haemodynamic instability[15]. Most affected children have recovered. Continued research is essential to understand the aetiology of the syndrome and ensure effective treatment. In the meantime doctors should remain vigilant. Signs and symptoms of the condition include: persistent fever, inflammation (neutrophilia, elevated CRP and lymphopaenia) and evidence of single or multi-organ dysfunction (shock, cardiac, respiratory, renal, gastrointestinal or neurological disorder) with additional features. This may include children fulfilling full or partial criteria for Kawasaki disease.
Development of therapeutics for SARS CoV2 Infection
Currently efforts are underway to develop licensed treatments for the whole age range in paediatrics. Children are enrolling into the ongoing RECOVERY trial in the UK, in which they may receive dexamethasone, azithromycin or lopinavir-ritonavir[16]. Remdesivir is available in the UK for patients aged 12 years and over[17] and trials are underway in younger children. There are also trials ongoing to study the effects of plasma[18], darunavir and cobicistat (in China), DAS181, nitric oxide (in patients aged 14 years and above), nebulized RLF-100 (aviptadil) in patients aged 12 years and above. Experimental treatments for PIMS-TS include corticosteroids, intravenous immunoglobulins, anakinra and tocilizumab[19,20].
Vaccines for COVID-19
Clinical trials have now started with several of the vaccines in development, such as Moderna’s mRNA-1273[23], Oxford University’s ChAdOx1 (now AZD1222)[24], Sinopharm’s BBIBP-CorV and CanSino Biologics Ad5-nCoV[25], and more recently the self-amplifying mRNA vaccine from Imperial College[26]. The early data released from these vaccine candidates, following their preclinical and Phase 1 studies, indicate a level of safety, as well as immunogenicity potential in stimulating both neutralising antibodies and T-cell responses. However, further proof of safety and efficacy in larger Phase 2 and 3 studies will be required before applications are made for authorisation by the regulators, and before public health immunisation programmes using these new vaccines can be initiated.
Vaccines in paediatric groups
Response to vaccines is dependent on the host, the pathogen and the vaccine itself. Age plays a key role with regards to the difference in response observed between infants and older children, so necessitating adjustments to the immunisation schedule for routine vaccines in children[12]. This is an evolving area of science, even for diseases such as influenza, where there has been recent recognition that vaccines used in infants may need adjuvants or a higher antigen content than previously thought, requiring efficacy trials in influenza lasting between 6 months and 3 years[27]. Therefore, designing vaccines for the paediatric population relies on an understanding of immune function by age group, the level of protection offered by different platforms, and the dosing regimen required. Currently available information includes a limited number of trials of vaccines in children (see Table 1). This is likely to expand as more data emerge from additional early trials in adults.
| ChAdOx1 (AZD1222) | Sponsor: Astra Zeneca/ Jenner Institute- Oxford | Phase 2 | 5-12 years | As part of a larger 10,260 participants study | Double-blind, randomised controlled trial |
| Lentiviral Minigene Vaccine (LV-SMENP) of COVID-19 Coronavirus | Sponsor: Shenzhen Geno-Immune Medical Institute | Phase 1-2 | 6 months-80 years | Open label, 100 subjects |
| COVID-19 Coronavirus Artificial Antigen Presenting Cell Vaccine | Sponsor: Shenzhen Geno-Immune Medical Institute | Phase 1 | Open label, 100 subjects |
Non-Pharmaceutical Interventions
As COVID-19 seems milder in children compared to the older population, general public health prevention strategies of frequent handwashing, social distancing and using masks in closed spaces are appropriate[28]. As winter approaches, with its associated risk of other respiratory viral diseases, wider use of influenza vaccine, which is reimbursed for healthy children in the UK up to 11 years old and for 11-18 year old children in at-risk groups, is advised. Increasing vaccination rates in children with underlying co-morbidities is a particular aim for the coming season[29].
Social impact of COVID-19 on education
Although children are not the most susceptible to COVID-19, they risk being amongst its biggest victims. In April 2020, the Secretary-General of the United Nations called on to leaders to “do everything in their power to cushion the impact of the pandemic and for children to be protected”[30]. The most significant effects of COVID-19 on children are related to widespread school closures leading to impacts on education and access to food through school. In addition, the safety of children may be compromised due to communities being in lockdown and the health of children may be affected because of a need to cut back on essential health expenditure and the suspension of childhood vaccination programs.
A total of 188 countries imposed national school closures, affecting more than 1.5 billion children and young adults[30]. School closures were based on evidence from previous influenza outbreaks, indicating
that reduced social contacts between students would interrupt transmission of coronavirus[31]. A recent systematic review of the effectiveness of school closures and other school social distancing practices during coronavirus identified a scarcity of policy-relevant data on the implementation of school social distancing and no data on ability to control transmission[32]. The authors recommended that policymakers need to be aware of the equivocal evidence when considering school closures for COVID-19.
Before this crisis, there was a sizeable difference in academic achievement between the poorest and richest children in the UK[33]. The effect of the COVID-19-related school shutdown in the UK seems to be disproportionately disrupting the education of those students who already have fewer advantages. For example, whilst 30% of pupils from middle-class homes are reported to be taking part in live and recorded lessons online every day, only 16% of working-class pupils do so. Furthermore, students from independent schools are more than twice as likely to take part in online lessons every day compared to their state-school counterparts[33]. It is also estimated that 700,000 students are not doing any schoolwork at home due to a lack of technology[34]. It has been recommended that during school closure, the UK government should help all children have the resources needed to access online learning, and disadvantaged pupils should have access to one-to-one or small group tuition[33].
Munro reported that children do not appear to be super-spreaders of SARS-CoV-2 transmission, with children unlikely to be the index case in households[9]. A collection of international family clusters found that children were responsible for around 10% of clusters[35] and a study from China found a lower rate of children as index cases (5%)[36]. Therefore, Munro recommended that governments worldwide should allow children back to school regardless of co-morbidities.
Conclusion
COVID-19 continues to have a huge impact on children and adults across the world. Fortunately, the disease presents less often and less severely in children. Although rates of critical illness are low, the development of therapeutics in this age group is important, especially considering the lack of long-term immunity to SARS-CoV-2 and potential for re-infection. The distinct PIMS-TS manifestation needs further review and consideration for therapeutic targets. The FPM Paediatrics and Other Vulnerable Populations Expert Group will continue to review the area and update this blog. There is some evidence of vertical transmission to neonates, although the route of transmission, neonatal and long-term effects are unclear[12]. This topic will be explored in a subsequent post by this group.
Have you enjoyed this article?
Please like and share our post on LinkedIn to help the article reach as many people as possible.
References
1. CDC COVID-19 Response Team. Coronavirus disease 2019 in children – United States, February 12-April 2, 2020. Morbid Mortal Weekly Report, 2020: 69: 422-6.
2. Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA 2020; 323: 1335.
3. Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. Published online 8 April 2020. https://doi.org/10.1001/jamapediatrics.2020.1346.
4. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020: 323: 1239-42.
5. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. Published online 6 July 2020. https://doi.org/10.1016/S0140-6736(20)31483-5.
6. Stringhini S, Wisniak A, Piumatti G. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. Published online 11 June 2020. https://doi.org/10.1016/S0140-6736(20)31304-0.
7. Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. Lancet Infect Dis. Published online 27 April 2020. https://doi.org/10.1016/S1473-3099(20)30287-5.
8. Royal College of Paediatrics and Child Health, Research & Evidence team. COVID-19 – research evidence summaries. Last modified 12 June 2020. https://www.rcpch.ac.uk/resources/covid-19-research-evidence-summaries (Accessed 16 June 2020).
9. Munro APS, Faust S. Children are not COVID-19 super spreaders: time to go back to school. Arch Dis Child 2020; 105: 618-9.
10. Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. Published online 25 June 2020. https://doi.org/10.1016/S2352-4642(20)30177-2.
11. Natale F, Ghio D, Tarchi D, et al. COVID-19 cases and case fatality rate by age. European Commission. Knowledge for Policy Briefing, 4 May 2020. https://ec.europa.eu/knowledge4policy/sites/know4pol/files/jrc120420_covid_risk_and_age.pdf (Accessed 20 July 2020).
12. Zimmermann P, Curtis N. COVID-19 in children, pregnancy and neonates: A review of epidemiologic and clinical features. Pediatr Infect Dis J 2020; 39: 469-77.
13. European Centre for Disease Prevention and Control. Paediatric inflammatory multisystem syndrome and SARS-CoV-2 infection in children. 15 May 2020. Stockholm: ECDC. https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-risk-assessment-paediatric-inflammatory-multisystem-syndrome-15-May-2020.pdf.
14. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a paediatric inflammatory syndrome temporally associated with SARS-CoV-2. JAMA 2020; 324:259-69.
15. Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. Published online 3 June 2020. https://doi.org/10.1136/bmj.m2094.
16. Randomised Evaluation of COVID-19 Therapy (RECOVERY). https://www.recoverytrial.net/files/recovery-protocol-v7-0-2020-06-18.pdf.
17. Veklury 100 mg. Summary of Product Characteristics. July 2020. Carrigtohill: Gilead Sciences Ltd. https://www.medicines.org.uk/emc/product/11597/smpc.
18. Convalescent Plasma in Pediatric COVID 19. NCT04458363. ClincalTrials.gov. NIH: U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04458363?recrs=a&type=Intr&cond=Covid+19&intr=plasma&age=0&draw=2&rank=1.
19. COVID-19 Therapeutics. April 2020. NIHR Innovation Observatory. http://www.io.nihr.ac.uk/report/covid-19-therapeutics/.
20. COVID-19 treatment and vaccine tracker. Milken Institute. https://covid-19tracker.milkeninstitute.org/#treatment_antibodies.
21. Ward, P et al. COVID-19/SARS-CoV-2 Pandemic. Faculty of Pharmaceutical Medicine blog, 6 April 2020. https://www.fpm.org.uk/blog/covid-19-sars-cov-2-pandemic/.
22. ICH guidelines. European Medicines Agency. Amsterdam. https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-guidelines/ich-guidelines.
23. Corbett KS, Darin Edwards D, Leist SR, et al. SARS-CoV-2 mRNA vaccine development enabled by prototype pathogen preparedness [Preprint]. bioRxiv Preprint Server for Biology. Published online 11 June 2020. https://doi.org/10.1101/2020.06.11.145920.
24. van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques [Preprint]. bioRxiv Preprint Server for Biology. Published online 13 May 2020. https://doi.org/10.1101/2020.05.13.093195.
25. Zhu F-C, Li Y-H, Guan X-H, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Published online 22 May 2020. https://doi.org/10.1016/S0140-6736(20)31208-3.
26. O’Hare R, Wighton K. Imperial to begin first human trials of new COVID-19 vaccine. 15 June 2020. London: Imperial College. https://www.imperial.ac.uk/news/198314/imperial-begin-first-human-trials-covid-19/.
27. Influenza vaccines – non-clinical and clinical module. European Medicines Agency. Amsterdam. https://www.ema.europa.eu/en/influenza-vaccines-non-clinical-clinical-module.
28. Coronavirus (COVID-19). Education and childcare. Guidance for teachers, school leaders, carers, parents and students. Department of Education, UK Government. https://www.gov.uk/coronavirus/education-and-childcare
29. Childhood flu programme: information for healthcare practitioners. Public Health England, UK Government. https://www.gov.uk/government/publications/childhood-flu-programme-qa-for-healthcare-professionals
30. Guterres A. Policy Brief: The impact of Covid-19 on children. 15 April 2020. United Nations. https://www.un.org/sites/un2.un.org/files/policy_brief_on_covid_impact_on_children_16_april_2020.pdf (Accessed 6 June 2020).
31. Jackson C, Vynnycky E, Mangtani P The relationship between school holidays and transmission of influenza in England and Wales. Am J Epidemiol 2016; 184: 644-51
32. Viner RM, Russel SJ, Croker H, et al. School closure and management practices during coronavirus outbreaks including COVID-19: a rapid systematic review Published online 6 April 2020. https://doi.org/10.1016/S2352-4642(20)30095-X.
33. Cullinane C, Montacute R. COVID-19 and social mobility impact brief #1: School shutdown. 6 April 2020. The Sutton Trust. https://www.suttontrust.com/our-research/covid-19-and-social-mobility-impact-brief/ (Accessed 6 June 2020).
34. Education Committee, Oral evidence: The impact of COVID-19 on education and children’s services. UK Parliament. https://committees.parliament.uk/work/202/the-impact-of-covid19-on-education-and-childrens-services/publications/ (Accessed 3 June 2020).
35. Zhu Y, Bloxham CJ, Hulme KD, et al. Children are unlikely to have been the primary source of household SARS-CoV-2 infections [Preprint]. medRxiv Preprint Server for Health Sciences. Published online 30 March 2020. https://doi.org/10.1101/2020.03.26.20044826.
36. Jing Q-L, Liu M-J, Yuan J, et al. Household secondary attack rate of COVID-19 and associated determinants [Preprint]. medRxiv Preprint Server for Health Sciences. Published online 15 April 2020. https://doi.org/10.1101/2020.04.11.20056010.
