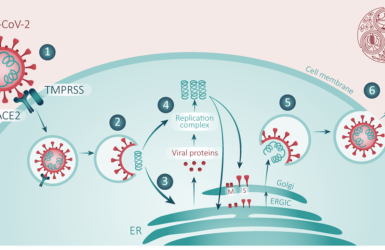
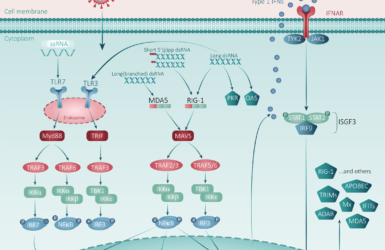
Does SARS-CoV-2 cause a vascular disease?
Posted on: Thursday 21 May 2020
Author: Professor Tim Higenbottam et al.
This article has been prepared by Professor Tim Higenbottam (FPM President), with input from Professor Anoop Chauhan (Portsmouth University), Professor Joseph Moellman (University of Cincinnati Ohio), Professor Jonathan Bernstein (University of Cincinnati Ohio), and Dr Wynne Weston Davies (Akari Therapeutics PLC)
It is provided for information and does not constitute advice or represent official FPM views or policy.
How to cite:
Higenbottam, T et al. (2020), ‘Does SARS-CoV-2 cause a vascular disease?’, Faculty of Pharmaceutical Medicine blog, 21 May 2020. Available at: https://www.fpm.org.uk/blog/does-sars-cov-2-cause-a-vascular-disease/ (Accessed: <date>).
Summary
Our understanding about the illnesses caused by SARS-CoV-2 has grown exponentially since December 2019. Use of histology, physiology, and the accompanying use of imaging of the lungs has demonstrated a new microvascular disease mechanism. This requires a different approach to respiratory support to restore normoxia and new therapeutics to halt the vascular lung and other organ damage plus reduce the excess systemic inflammatory response. The start of vascular damage is difficult to define but admission to a hospital in the UK, as a result the symptom of breathlessness and/or measurement of hypoxaemia (1) is useful. It equates with grade 4 on the WHO ordinal scale (2). The histopathology shows evidence of a thrombotic microangiopathy (TMA) in the lungs, that has also been seen in the systemic circulation. While treatments with antiviral agents may have a therapeutic impact (3), specific remedies are needed for management of TMA once this inflammatory stage is clinically confirmed.
The Pneumonia of SARS-CoV and MERS-CoV compared with SARS-CoV-2
Other than supportive measures, there is a lack of approved treatments for the breathless/hypoxic stage of the SARS-CoV-2. Note that this is also the case for the earlier SARS‐CoV and MERS-CoV pneumonias. However, it is encouraging that several diverse therapeutic approaches are being tested.
Our knowledge about this breathlessness/hypoxia phase has improved since December 2019 but remains limited. CXR and CT scans of the lungs offer some degree of recognition but are relatively non-specific (4,5). Histological studies have been limited because of the risk of infection, which has limited large scale studies of the corona viral pneumonias. The handling of pathology specimens needs to be rigorously managed (6).
The pulmonary histological features of pulmonary SARS‐CoV and MERS-CoV infections have been described. The cardinal inflammatory findings include acute diffuse alveolar damage (DAD) with later development of fibrinous and organising pneumonia. This progresses to type II pneumocyte hyperplasia, and predominantly interstitial lymphocytic pneumonia, often associated with multinucleate syncytial cells. All these are common features of acute respiratory distress syndrome (ARDS) caused by several lung injuries and infections. Autopsy has revealed very heavy swollen oedematous lungs (7,8).
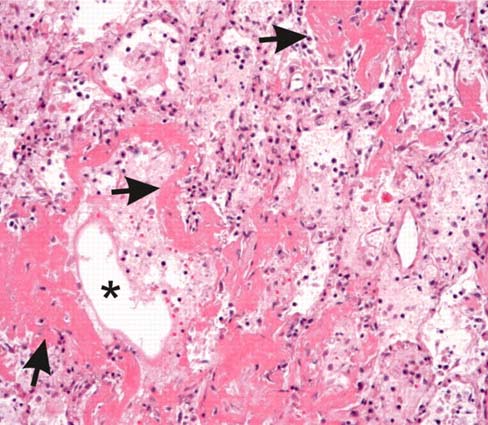
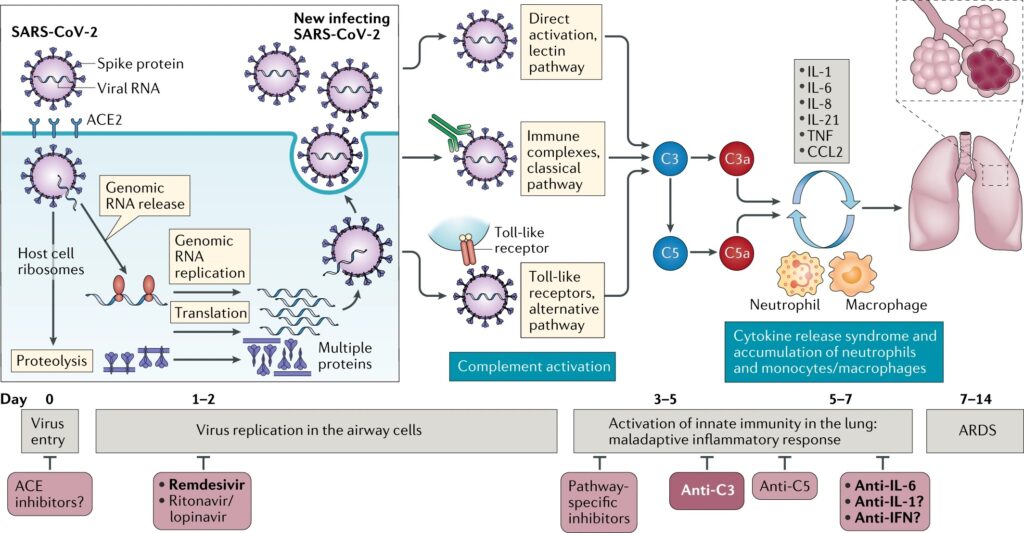
SARS-CoV-2 pneumonia exhibits a thrombotic microangiopathy through complement activation by the alternative and lectin-based pathways
A different lung pathology has been reported in SARS-CoV-2 pneumonia, where there is distinct absence of the ARDS classical features described above. Post-mortem histology shows there was much less inflammation associated with extensive damage to the alveolar structures and relatively small amounts of exudate in the alveoli with some hyaline membrane formation. Two additional observations made in early studies were the presence of dilated proximal pulmonary arteries and extensive microthrombi in alveolar wall capillaries (8). Similar findings of diffuse alveolar damage (DAD) in association with capillary micro thrombosis have also been recently reported in deceased patients studied at autopsy in the United States (10).
Investigators have studied lung biopsies from two deceased patients, one of which didn’t receive mechanical ventilation, while the other did until death (11). Unlike the earlier corona virus pneumonias, in the unventilated patient the DAD and hyaline membrane deposition findings were absent and only the presence of limited inflammation and type II pneumocyte hyperplasia were found. In contrast, the ventilated patient exhibited areas of DAD with limited inflammation. However, both patients had widespread alveolar septal capillary injury with significant septal capillary mural and luminal fibrin deposition and permeation of the interalveolar septa by a few neutrophils. The most prominent histological finding for both patients was the presence of microvascular thromboses involving the capillaries.
These pulmonary findings were accompanied by significant deposits of terminal complement components C5b-9, the membrane attack complex (MAC), C4d, and mannose binding lectin (MBL) and mannose-associated serine protease (MASP)2. This is consistent with sustained, systemic activation of the alternative and lectin-based complement pathways.
These pathological changes share very similar features to complement induced thrombotic microangiopathy (TMA) as seen in atypical Haemolytic Uraemia syndrome (aHUS), the antiphospholipid syndrome (APS) C3 glomerulopathy (C3G) and post-haematopoietic stem cell transplant (HSCT-TMA). These four causes of TMA are all now known to be associated with the terminal complement pathway dysregulation. Anticomplement C5 therapy is now approved, specifically eculizumab a monoclonal antibody therapy, has already gained Market Authorisation for these illnesses. It is, along with other C5 inhibitors, being considered for treatment of SARS-CoV-2 pneumonia in conjunction with associated systemic disease.
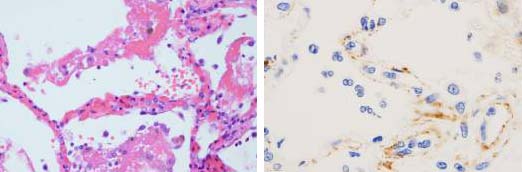
Within the alveolar septa it was found that with the SARS-CoV-2 particles, unlike the former Corona viral pneumonias, there were no cellular viral cytopathic changes. This perhaps indicates that with the CoV pneumonias, unlike SARS-CoV and MERS, complement activation was the cause of the vascular injury.
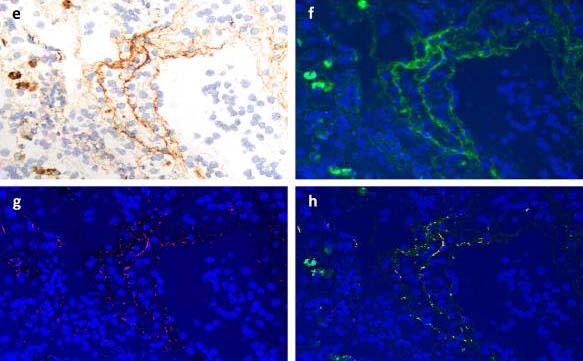
Similar complement mediated microvascular injury in systemic blood vessels
There are indications that the microvascular pathology associated with SARS-CoV-2 is not confined to the lungs. These patients also showed similar histopathologic findings in the skin. Three SARS-CoV-2 pneumonia patients with elevated d-dimer levels had skin biopsies which showed an absence of inflammation but extensive vascular deposits of C5b-9, C3d, and C4d throughout the dermis with marked deposition in an occluded artery (11). These observations may explain the systemic diseases associated with SARS-CoV-2 being reported. For example, cases of systemic arterial disease resembling Kawasaki disease have been observed in children with SARS-CoV-2 infection (12).
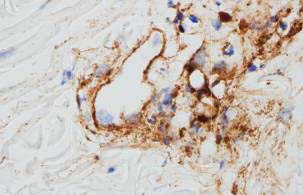
Complement initiated TMA in lung transplant rejection
There is a precedent for terminal pathway dysregulation causing pulmonary TMA. An early report by Magro et.al. in 2011 found a very similar histological appearance in lung transplant patients, who had been well managed with immunosuppressants but suddenly became breathless (13). Transbronchial biopsies of the lungs were significant for septal capillary necrosis with C1q, C3, C4d, and/or C5b-9 and immunoglobulin (including IgG) deposition in the septal capillaries and associated endothelial cells. The degree of septal capillary necrosis and C1q, C3, C4d, and C5b-9 deposition was significantly less in post transplantation patients who were clinically doing well (P<0.0001). Indirect anti-endothelial cell antibody studies were positive in most patients. The presence of C1q had not been sought in the later studies of SARS-CoV-2 pneumonia, however the finding could support the deposition that the classical complement pathway is also involved in this transplant lung TMA disease.
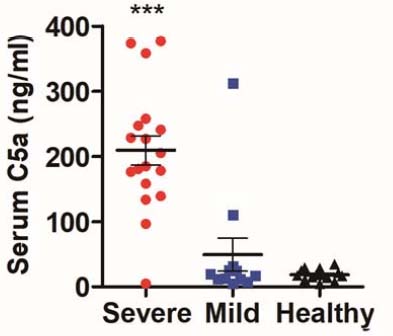
Extensive studies of SARS-CoV-2 patients by Gao and colleagues (9) reported high circulating levels of C5b-9, the terminal marker for complement activation, in SARS-CoV-2 patients with pneumonia. Furthermore, a trial of a mouse hybrid antibody therapy to C5 in four of these patients resulted in clinical improvement. It has been proposed that measurement of C5b-9 in th blood could be used as clinical guide to the start of the vascular disease.
The impact of the TMA on lung respiratory function
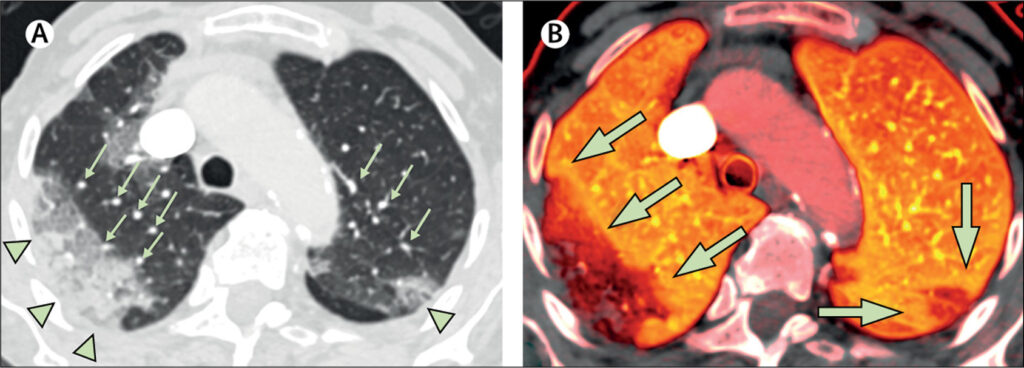
A proportion of SARS-CoV-2 pneumonias have an unusual pathology characterized by TMA and dilated pulmonary arteries (8,10). Zhou and Gatinnoni (14,15) have reported 70% of the SARS-CoV-2 pneumonia patients usually have high or normal lung compliance. In contrast to acute lung injury (ALI) or DAD, their lungs are not “stiff” but are easily inflated. However, there is evidence of extensive pulmonary ventilation and perfusion mismatch (Va/Q) with markedly increased dead space greater than the usual 150 mls observed for an adult male. In addition, there is significant right to left shunting of blood that causes profound hypoxia. Gatinnoni and colleagues used the calculation of Hounsfield numbers from patient CT scans to illustrate that the large areas of under perfused lung regions resulted in the observed enlarged dead space (15).
It is now possible to image the pulmonary circulation for thrombi and assess the degree of lung perfusion using Dual Energy CT scans with iodine perfusion, a standard for the diagnosis of pulmonary emboli. A small study of three patients with RT-PCR confirmed SARS-CoV-2 pneumonia without pulmonary emboli and no history of asthma or COPD showed the large areas of aerated but under perfused lungs associated with dilated blood vessels (16), confirming Gatinnoni et al. observations.
Gatinnoni makes the point that current CPAP and BiPAP are not necessarily of value in these patients, as they raised intra-thoracic pressure further which can further limit the perfusion to well ventilated areas of the injured lungs.
Treatments for TMA
The clinical management of diseases like atypical haemolytic uremic syndrome (aHUS), C3 glomerulopathy (C3G), antiphospholipid syndrome (APS) and post-haematopoietic stem cell transplant (HSCT-TMA) associated with TMA caused by complement dysregulation has included the use of C5 inhibitors (17). All these diseases have terminal complement pathway dysregulation with increased C5b-9 similarly to what was reported in SARS-CoV-2 pneumonia (18).
Eculizumab, which is an anti- C5 monoclonal antibody, is approved for treatment of aHUS, PNH and C3G (19). Individuals presenting with TMA who fail to respond to eculizumab have been demonstrated to have genetic variants in the non-complement genes DGKE, INF2 and MMACHC (20). A rare polymorphism in C5 (p.R885H) has been reported in the Japanese population which prevented eculizumab binding (21). More recently, a European patient with a functionally significant CFH mutation (p.D1119G) who developed TMA post-bone marrow transplant, was shown to carry this SNP preventing the use of eculizumab (22). In this case, an alternative C5 inhibitor, Coversin (now called nomacopan), has been used as it binds to a different epitope on C5 (23).
Treatment with complement C5 and C5a inhibitors of transplant associated TMA (TA-TMA) especially in children post-hematopoietic stem cell transplants, has been found to significantly reduce morbidity and mortality in these patients (24).
Future treatments for the terminal complement pathway dysregulation of SARS-CoV-2 pneumonia
Although experience is limited, eculizumab has been introduced for compassionate use in SARS-CoV-2 infections in four patients and has been found to be efficacious and safe. All four patients were admitted to the hospital with rapidly worsening respiratory function and evidence of ground-glass bilateral lung infiltrates on chest CT-scans. All showed a marked clinical improvement within the first 48 hours after the first dose of eculizumab, including an elderly woman with arterial hypertension, COPD, and cardiovascular disease (25).
Alternative therapies for terminal pathway dysregulation have been developed since complement C5 polymorphisms can limit eculizumab’s effectiveness. For this reason complete complement blockade should be confirmed before the continued use of these therapies (21). To circumvent this problem, several antagonists are currently in clinical trials. They include Ravulizumab, an optimized monoclonal C5 antibody (Alexion; NCT02946463, NCT03056040), Zilucoplan, a synthetic peptide that inhibits C5 (RaPharma NCT03030183, NCT03078582), Narsoplimab, an antibody targeting the MBL pathway (Omeros Corporation OMS721), and ALN-CC5, an RNAi that interferes with C5 gene expression (NCT02352493 Alnylam). Nomacopan (Coversin) (NCT02591862; Akari) is a recombinant protein derived from the tick, Ornithodoros moubata. It binds to a different epitope on C5, thereby completely blocking the terminal pathway. This molecule has the added advantage of blocking leukotriene B4 activity that has additional anti-inflammatory effects and is an important chemotactic factor for neutrophils [22] [23]. Roche and Chugai have developed SKY/RO7112689, a molecule which works in patients with the C5 variant p. Arg885His and has long-lasting C5 inhibition properties (25). There are also new products in development by Novartis (LFG316) and Regeneron (puzelimab/REGN3918) for the treatment of Hemolytic anaemia, paroxysmal nocturnal Hemoglobinuria (PNH).
An emerging theory that multiple variations in complement associated genes account for the higher risks seen with black and minority ethnic (BAME) patients with SRAS-CoV-2 infections
There is a strong association between the risk of developing complement driven TMA in people receiving hemopoietic stem cell transplants (HSCT) and the number of genetic variations in the complement genes. Earlier work from the complement genetics studies in haematopoietic stem cell transplantation (HSCT) recipients has provided evidence that African Americans compared to White Americans have an increased risk for developing TMA and a higher risk of mortality (27,28). This is believed to be due to the increased numbers of complement gene polymorphisms in African Americans that react with stronger complement activation response to infection with highly pathogenic coronaviruses such as SARS-COV-2, resulting in uncontrolled pulmonary tissue inflammation and complement deposition in several organs (29,30).
Conclusions
Currently, there are no approved treatments for the thrombo-Inflammatory stage of the SARS-CoV-2 pneumonia and the current support measures are not able to avoid mechanical ventilation of patients.
As with all newly recognized diseases it takes time to develop medicines and devices that provide optimal care. This time needs to be spent learning from small studies using novel therapies that target specific pathways such as complement to see if they can safely influence clinical outcomes in SARS-CoV-2 pneumonia patients. The Agile Accord studies seem to offer a way forward to simultaneously compare several novel medicines. This new way of undertaking First into Human (FiH) studies could speed the way to phase III studies.
However, only when safety, appropriate posology and dose are known, can large randomized double-blind studies be initiated. Priority needs to be given to innovative approaches that can improve care of SARS-CoV-2 patients as this new virus is significantly more virulent than its earlier predecessors. The future remains uncertain, but we are beginning to see a path forward.
References
1. NHS England and NHSI Clinical management of persons admitted to hospital with suspected COVID-19 infection. https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/clinical-management-of-persons-admitted-to-hospita-v1-19-march-2020.pdf
2. WHO R&D Blueprint COVID-19 Therapeutic Trial Synopsis. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf
3. Cao B, Wang Y, Wen D, et al, A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med 2020; 382: 1787-99. DOI: 10.1056/NEJMoa2001282
4. Zhao W, Zhong Z, Xie X, et al. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. American Journal of Roentgenology. 2020;214: 1072-1077.
5. The continuing evolution of COVID-19 imaging pathways in the UK: a British Society of Thoracic Imaging expert reference group update. Clinical Radiology 2020 published on-line https://doi.org/10.1016/j.crad.2020.04.002
6. Hanley B, Lucas S B, Esther Youd E, Swift B, Osborn M. Autopsy in suspected COVID-19 cases. J Clin Pathol 2020; 73: 239–242.
7. Liu J, Zheng X, Tong Q, et al, Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS‐CoV, MERS‐CoV, and 2019‐nCoV. Med Virology. 2020; 92: 491-494.
8. Xiaohong Y, Tingyuan L, Zhicheng H et al. New coronavirus pneumonia (COVID-19) 3 cases of multiple-site puncture histopathological pathology of human remains Journal of Chinese Pathology, 2020,49: Online Pre-publish. DOI: 10.3760/cma.j.cn112151-20200312-00193.
9. Gao T, Hu M, Zhang X, et al, Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation.
10. Fox SE, Akmatbekov A, Harbert, J L, et al. Pulmonary and Cardiac Pathology in Covid-19: The First Autopsy Series from New Orleans. 2020; April. medRxiv preprint doi: https://doi.org/10.1101/2020.04.06.20050575.
11. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Translational Research. April 2020. doi: 10.1016/j.trsl.2020.04.007.
12. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P, Hyperinflammatory shock in children during COVID-19 pandemic. Lancet published on-line May 6, 2020 https://doi.org/10.1016/S0140-6736(20)31094-1.
13. Magro CM, Deng A, Pope-Harman A, et al. Humorally Mediated Posttransplantation Septal Capillary Injury Syndrome as a Common Form of Pulmonary Allograft Rejection: A Hypothesis. Transplantation 2002; 74: 1273–1280.
14. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395: 1054–62.
15. Gattinoni L, Chiumello D, Pietro Caironi P, et al, COVID-19 pneumonia: different respiratory treatment for different phenotypes? (2020) Intensive Care Medicine; DOI: 10.1007/s00134-020-06033-2.
16. Lang M, Som A, Mendoza DP et al, Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis 2020 Published Online April 30, 2020 https://doi.org/10.1016/S1473-3099(20)30367-4
17. Wong E K S, David Kavanagh D, Diseases of complement dysregulation—an overview. Seminars in Immunopathology (2018) 40:49–64. https://doi.org/10.1007/s00281-017-0663-8.
18. Risitano A M, Mastellos D C, Huber- Lang M, et al, Complement as a target in COVID-19? Nature Reviews Immunology https://doi.org/10.1038/s41577-020-0320-7 19. Eculizumab- SmPC https://www.medicines.org.uk/emc/product/362.
20. Beck BB, van Spronsen F, Diepstra A, Berger RM, Komhoff M. Renal thrombotic microangiopathy in patients with cblC defect: review of an under-recognized entity. Pediatr Nephrol 2017; 32: 733–741.
21. Ozaltin F, Li B, Rauhauser A, An SW, Soylemezoglu O, Gonul II et al. DGKE variants cause a glomerular microangiopathy that mimics membranoproliferative GN. J Am Soc Nephrol 2013: 24: 377–384.
22. Nishimura J, Yamamoto M, Hayashi S, Ohyashiki K, Ando K, Brodsky AL et al. Genetic variants in C5 and poor response to eculizumab. N Engl J Med 2014; 370: 632–639.
23. Goodship T, Pinto F, Weston-Davies W, Silva J, Nishimura J, Nunn M et al. Use of the complement inhibitor coversin to treat HSCT-associated TMA. Blood Adv 2017; 1:1254–1258. 24. Dvorak C, Higham C, Shimano K A. Transplant-Associated Thrombotic Microangiopathy in Pediatric Hematopoietic Cell Transplant Recipients: A Practical Approach to Diagnosis and Management. Front. Pediatr., 09 April 2019 https://doi.org/10.3389/fped.2019.00133
25. DIURNO F, NUMIS F G, PORTA G, et al, Eculizumab treatment in patients with COVID-19: Eculizumab treatment in patients with COViD-19preliminary results from real life ASL Napoli 2 Nord experience. European Review for Medical and Pharmacological Sciences. 2020; 24: 4040-4047.
26. Fukuzawa T, Sampei Z, Kenta Haraya K, et al, Long lasting neutralization of C5 by SKY59, a novel recycling antibody, is a potential therapy for complement-mediated diseases. Nature Scientific Reports. 2017 7: 1080 | DOI:10.1038/s41598-017-01087-7
27. Jodele S, Koehl J, Tackling COVID-19 infection through complement-targeted immunotherapy. Authorea. 2020 May 06, 020.https://DOI: 10.22541/au.158880110.01220133
28. Baker KS, Davies SM, Majhail NS, Hassebroek A, Klein JP, Ballen KK, et al. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant 2009; 15: 1543-1554.
29. Paun CC, Lechanteur YTE, Groenewoud JMM, Altay L, Schick T, Daha MR, et al. A Novel Complotype Combination Associates with Age-Related Macular Degeneration and High Complement Activation Levels in vivo. Sci Rep 2016; 6: 26568.
30. Jodele S, Davies SM, Lane A, Khoury J, Dandoy C, Goebel J, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood 2014; 124: 645-653.
About the authors
Professor Tim Higenbottam is an (unpaid) advisor to Akari Therapeutics PLC
Prof Anoop Chauhan MB ChB PhD is a Professor Respiratory Medicine at Portsmouth University
Professor Joseph Moellman MD is an Associate Professor of Emergency Medicine at the University of Cincinnati Ohio
Professor Jonathan Bernstein MD is a Professor of Clinical Medicine at the University of Cincinnati Ohio
Dr Wynne Weston Davies MB BS FRCS is the Medical Director of Akari Therapeutics PLC.
Copyright notice
Any redistribution or reproduction of part or all of the contents in any form is prohibited other than the following:
- you may print or download to a local hard disk extracts for your personal and non-commercial use only
- you may copy the content to individual third parties for their personal use, but only if you acknowledge the website as the source of the material
You may not, except with our express written permission, distribute or commercially exploit the content. Nor may you transmit it or store it in any other website or other form of electronic retrieval system.
Please contact us if you have any queries.