FPM statement on COVID-19 vaccine dosing regimen and roll-out
Posted on: Thursday 28 January 2021
Author: FPM
- The Faculty of Pharmaceutical Medicine (FPM) welcomes the collaboration between the pharmaceutical industry, academia, clinicians, charities, the NHS and the public in developing vaccines contributing towards a solution to the COVID-19 pandemic.
- The Commission on Human Medicine (CHM), Joint Committee on Vaccination and Immunisation (JCVI) and Medicines and Healthcare products Regulatory Agency (MHRA) have reviewed the public data and the technical dossiers on two COVID-19 vaccines (the BioNTech /Pfizer and Oxford/AZ vaccines), and have formed recommendations based on these data. It is their considered opinion and made in the interests of public health.
- FPM recognises that there may be disagreement between experts on the analysis and the recommendations. FPM notes that it is the competent bodies’ responsibility in particular the JVCI to advise on the emergency use of these two vaccines on behalf of the nation.
- FPM recognises the difficulties of rapidly rolling out vaccines to all high risk patients, when supply is constrained.
- Pharmaceutical companies routinely expect their products to be administered in line with the clinical trial data submitted to regulatory authorities. The FPM recognises the unprecedented situation of the pandemic and accordingly understands that the available data may also need to be weighed against other public health considerations. This may influence the expert recommendations on the use of vaccines in practice.
- Pharmaceutical companies have a responsibility to operate a comprehensive pharmacovigilance system for their products and to continuously evaluate the benefit risk profile in conjunction with regulatory authorities.
- FPM recommends ongoing active monitoring of the roll-out with collaboration between all stakeholders as recommended by the WHO. The PHE COVID-19 vaccine surveillance strategy is one key aspect, together with ongoing monitoring by companies and by the MHRA. It is particularly important to learn about the impact of vaccines on transmissibility of the virus.
- FPM notes that principles of maintaining patient trust and of obtaining valid consent prior to any medical intervention, remain critically important during a public health emergency.
Read our COVID-19 blog articles

It’s All Greek to Me: SARS-CoV-2 Variants, Vaccines and Antivirals
A Deep Dive by Professor Penny Ward et al
FPM Statement on COVID Vaccine Supply May 2021
FPM urges governments, industry and regulators to collaborate to find solutions to ensure supply as soon as soon as possible.
Inhalation therapies for COVID-19
Can we inhibit the initial stages of the infection of the respiratory tract?

Pregnancy, COVID-19 and Emerging Therapeutic Options
Allyah Abbas-Hanif, Sue Tansey, Sankarasubramanian Rajaram, Penny Ward

FPM Clinical Trials Resilience Survey – Report Published
Adaptations and innovations made to clinical trial programmes and regulatory frameworks during the COVID-19 pandemic.

Blog article: Preparedness Planning on Public Health Emergencies (PHEs)
The webinar series that has brought FPM into the heart of the COVID-19 pandemic.
What you need to know about COVID-19 testing
An article by Robert L Holland FFPM and John Bagshaw of the British In Vitro Diagnostics Association

Press release: Faculty of Pharmaceutical Medicine launches ‘Principles for Clinical Studies into COVID-19’ guidance
Read the press release

COVID-19 – how pharmaceutical medicine is meeting the challenge
A report of the FPM survey of members on the effect of COVID-19 on their professional lives
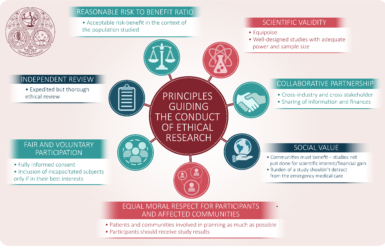
The ethics of conducting clinical trials in the search for treatments and vaccines against COVID-19
Some of the important ethical issues when planning and conducting clinical trials in the COVID 19 pandemic.
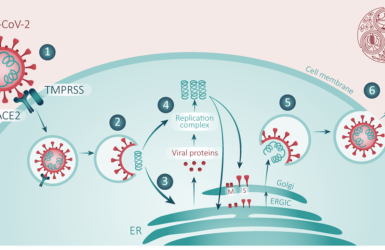
COVID-19/SARS-CoV-2 Pandemic
A landmark review by Dr Penny Ward et al addressing current knowledge concerning SARS-CoV-2 and COVID-19 disease.