First results from the PANORAMIC Study: Molnupiravir
Posted on: Tuesday 1 November 2022
Author: Penny Ward

Introduction
In the FPM Blog published in August, the use of antiviral treatment starting within five days of first onset of symptoms was mooted to prevent hospitalisation and death from COVID in high-risk subjects1. At the time of writing, molnupiravir was thought to potentially reduce hospitalisation and death by ~30% based on the results of the multinational multicentre double-blind randomised placebo controlled Phase III study MOVe-OUT. This study compared outcomes of usual care plus treatment with 800mg molnupiravir twice daily for five days starting within five days of symptom onset to usual care alone in non hospitalised patients with at least one risk factor for severe outcomes. This was a trial in two parts: an interim analysis based on the first 775 subjects randomised demonstrated that the incidence of all cause hospitalisation/death was reduced from 53/377 subjects (14.1%) in the group receiving usual care to 28/385 subjects receiving molnupiravir (7.3%) representing an approx~50% reduction in hospitalisations/deaths among molnupiravir recipients. However, in the final analysis of 1408 subjects included in the modified intent to treat population, 68/699 (9.7%) of those receiving usual care died/were hospitalised compared to 48/709 ( 6.8%) of those given molnupiravir resulting in an approx~30% reduction in all cause hospitalisation/death within 28 days. Of the ten reported deaths, one was in the group receiving molnupiravir and nine in the group receiving usual care2. This trial was conducted when the Delta variant was the dominant clade in circulation. As it started prior to the availability of vaccines, the recruited population included only subjects that had not been vaccinated and who had at least one high risk factor for more severe disease/death from COVID based on the epidemiology to that point. COVID vaccines became available in late 2020 and at the time molnupiravir received conditional approval in the UK the majority of the high-risk population here were already vaccinated. A key question remaining was whether this or other antiviral agents would add value beyond vaccination alone in reducing the rate of hospitalisation/death from COVID among high-risk patients. Accordingly, the PANORAMIC study was set up to address this.
Results
The PANORAMIC study3 recruited previously vaccinated patients with PCR or lateral flow test positive COVID breakthrough infection, with symptoms not exceeding five days, who were either aged over 50 years of age with or without additional risk factors or were aged 18-49 years with one of the following known underlying chronic health conditions considered to make them clinically vulnerable:
- chronic respiratory disease (including chronic obstructive pulmonary disease (COPD), cystic fibrosis and asthma requiring at least daily use of preventative and/or reliever medication)
- chronic heart or vascular disease
- chronic kidney disease
- chronic liver disease
- chronic neurological disease (including dementia, stroke, epilepsy)
- severe and profound learning disability
- Down’s syndrome
- Diabetes mellitus (Type or Type II)
- immunosuppression: primary (e.g., inherited immune disorders resulting from genetic mutations, usually present at birth and diagnosed in childhood) or secondary due to disease or treatment (e.g., sickle cell, HIV, cancer, chemotherapy)
- solid organ, bone marrow and stem cell transplant recipients
- morbid obesity (BMI >35)
- severe mental illness
- care home resident
- judged by recruiting medically qualified professional, research nurse, nurse prescriber, prescribing pharmacist, dependent on the ISA for the specific IMP involved, to be clinically vulnerable
Patients were randomised in an open label fashion to receive usual care plus 800mg molnupiravir to be taken twice daily for five days starting within five days of symptom onset or to usual care alone. Recruitment began in December 2021 when the omicron variant was rapidly becoming the dominant viral strain. The initial sample size required was estimated to be ~5300 subjects per group based on the incidence rate of hospitalisations/deaths observed in a separate UK study, PRINCIPLE. However, it was becoming clear by this point that successive waves of infection caused by more recent variants were less likely to result in hospitalisation and death, particularly in vaccinated patients, so the protocol allowed an increase in sample size to reflect this. A total of 25,000 randomised subjects provided data for the primary endpoint analysis investigating the efficacy of molnupiravir. Of the randomised population, 94% had received at least three vaccine shots, ~25% were aged 65 or older, and the majority of patients reported minor symptoms. Only fatigue, feeling generally unwell and cough symptoms were reported as being of moderate or greater severity in more than 30% of subjects in each group3.
The rate of hospitalisation and death in the study was significantly lower, in both treatment groups, than the incidence observed in an unvaccinated population in the MOVe-OUT study. Among subjects receiving only usual care, 96/12484 (0.77%) subjects required hospitalisation or died compared to 103/12516 (0.82%) in the molnupiravir treated group. The outcomes suggest that molnupiravir treatment may not confer a benefit in terms of reducing progression to more severe disease in vaccinated high risk patients with break through infection caused by the more recent viral strains which cause less severe disease than the Delta and other variants circulating previously. Does this mean there was no benefit to trial participants at all? By no means, firstly treatment with molnupiravir significantly reduced the time to first recovery from a median of 14.5 days in the usual care group to 10.3 days in the molnupiravir treated population, a feature better demonstrated graphically3.
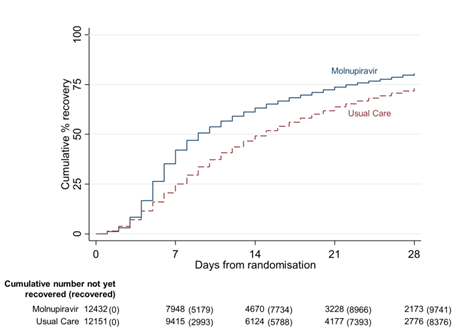
Additionally, molnupiravir treatment increased the proportion of patients without detectable virus in nose/throat swabs at Day 7 from 3% in the placebo group to 21% and also significantly decreased viral burden2. All of these features are benefits to patients who will feel better faster and thereby likely to be more quickly able to return to work/usual activities. This in turn can provide benefits in essential industries, not least healthcare, where COVID illness-related time away from work has exacerbated existing personnel shortages. In addition, the more rapid clearance of virus and lower viral burden may reduce the risk of transmission to others. The study team are continuing to follow treated patients to assess potential for reduced potential for long covid symptoms and a report is anticipated at a later date.
Subgroup analyses of the primary outcome suggest that there may be some differences in older patients with point estimates demonstrating a potential reduction in rate of hospitalisations/deaths in the over 65-year old group receiving molnupiravir2. This has also been reported in a retrospective observational comparison from Israel3, and could merit further investigation as it is the frailer older population that are currently at greatest risk of needing hospital care. In addition, five deaths were noted among placebo recipients compared to two in the molnupiravir treated group. This feature also merits some additional discussion. As the data are reported in preprint format, it is hoped to see more clarity on these topics in the final publication when available.
Safety
Data on safety is not reported in detail although the product was reportedly well tolerated with ~1% of subjects withdrawing from treatment for adverse effects. A more complete description of these would be useful to enable comparison to data previously reported2.
What next?
The results of this study need to be considered in the light of the evolving pattern of COVID illness, which has become milder as variant succeeds variant. Other groups have reported greater benefit in older (aged >65) patients4 and this might also be the case in PANORAMIC and would merit further investigation in a frail elderly group such as a care home population. In the meantime, while clearly disappointing in terms of use of this agent to prevent more severe disease in many, the faster rate of recovery and reduction in viral burden is nonetheless a benefit, particularly for workers in essential industries where an earlier return to work could reduce the impact of manpower shortages. Food for thought.
References
- Ward, P (2022), ‘How can we deploy the available toolkit to reduce the burden of respiratory viral infections on the NHS in 2022-3?’, Faculty of Pharmaceutical Medicine, 5 August 2022. Available at: https://www.fpm.org.uk/blog/how-can-we-deploy-the-available-toolkit-to-reduce-the-burden-of-respiratory-viral-infections-on-the-nhs-in-2022-3/
- Jayk Bernal A, Gomes da Silva MM, Musungaie DB et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. 2022; 386: 509-520. doi: 10.1056/NEJMoa2116044. Epub 2021 Dec 16. PMID: 34914868; PMCID: PMC8693688.
- Butler C, Hobbs R, Gbinigie O et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): preliminary analysis from the United Kingdom randomised, controlled open-label, platform adaptive trial. 4 October 2022. Preprint available at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4237902
- Arbel R, Sagy, Battat E. (2022). Molnupiravir Use and Severe Covid-19 Outcomes During the Omicron Surge. 10.21203/rs.3.rs-2115769/v1.
More from the FPM blog

How can we deploy the available toolkit to reduce the burden of respiratory viral infections on the NHS in 2022-3?
New blog by Penny Ward

Professor Penny Ward wins FPM Volunteer Award
From July 2022
Professor Ward won our brand new award for her services to FPM

It’s All Greek to Me: SARS-CoV-2 Variants, Vaccines and Antivirals
A Deep Dive by Professor Penny Ward et al
A Year with the ‘First Horseman’
Penny Ward reflects on what we have learned one year after the WHO announced the start of the COVID-19 pandemic.

Pregnancy, COVID-19 and Emerging Therapeutic Options
Allyah Abbas-Hanif, Sue Tansey, Sankarasubramanian Rajaram, Penny Ward

The End of the Transition Period and Recent announcements from the MHRA
What do you need to know for 1 January 2021?