A Year with the ‘First Horseman’
Posted on: Thursday 8 April 2021
Author: Prof Penny Ward
This article has been prepared by Prof Penny Ward FFPM.
It is provided for information and does not constitute advice or represent official FPM views or policy.
How to cite:
Ward, P (2020), ‘A Year with the ‘First Horseman’, Blog of the Faculty of Pharmaceutical Medicine, 8 April 2021. Available at: <link> (Accessed: <date>).
A Year with the ‘First Horseman’1.

We are now one year after the WHO announced the start of the COVID-19 pandemic and it is appropriate to pause and reflect on what we have learned over this time. Have we got control of the disease or what more needs to be done to keep control and reduce disease impact on individuals and the economy as we start the path out of isolation?
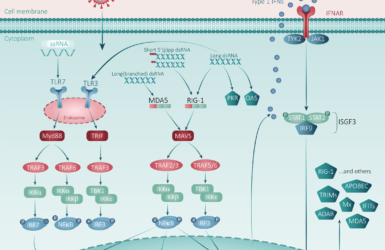
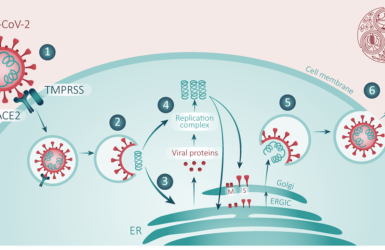
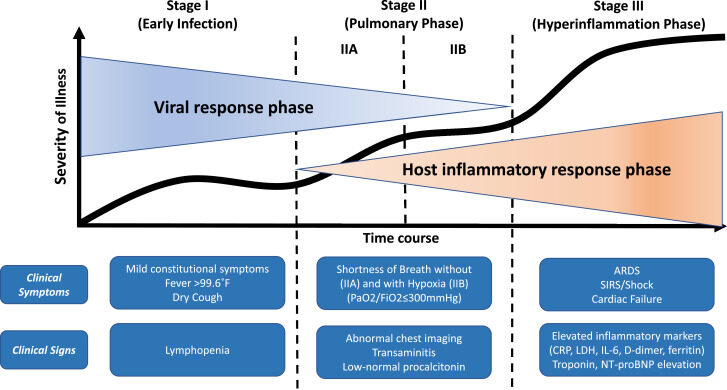
This time last year the FPM published our first blog on COVID. Looking back at that first summary, the advances made in our understanding of disease pathogenesis are clear. Although COVID begins as a respiratory viral infection, the disease then proceeds in phases. The initial phase is characterised by rapidly rising viral titres in respiratory secretions leading to pulmonary inflammation and a later hyperinflammatory phase dominated by progressive multiorgan system dysfunction which is the main cause of death (Figure 1)2. This pattern of disease suggests avenues for care both in the community (to reduce disease spread, reduce morbidity and prevent need for hospitalisation) and in hospital (to reduce mortality).
Hospital Care
Given the pathogenesis of this disorder, it is not surprising that anti-inflammatory treatments (corticosteroid, IL6 inhibitors) are, to date, the only pharmaceutical therapies which have reduced disease mortality in hospitalized patients, particularly among patients requiring oxygen supplementation3,4,5. In addition, prophylactic anticoagulation is recommended to limit thrombosis resulting from the widespread coagulopathy which accompanies endothelial inflammation6,7. Additional support may be required in the event of organ failure such as renal replacement therapy and Extra-Corporeal Membrane Oxygenation (ECMO) for terminal respiratory failure. These approaches have contributed to a blunting of the mortality rate among hospitalised patients, although the 28 day mortality rate may also be affected by the rate of hospital admissions and the proportion needing admission to the critical care unit (Figure 2)8.
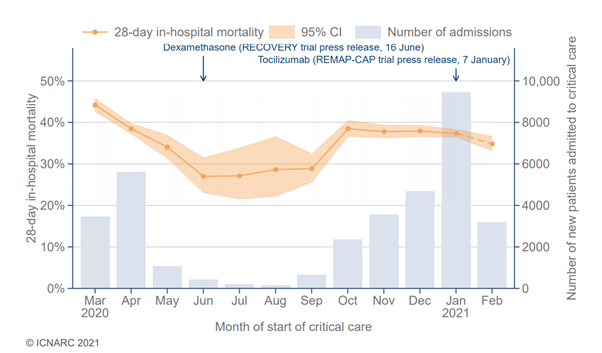
The early decline in 28 day mortality during the first wave was associated with rapid learning on the limited efficacy of early invasive mechanical ventilation (IVM) in reducing the mortality rate in patients with respiratory failure. Decline in the use of invasive ventilation during the first wave was associated with improved survival and a switch to the use of high flow nasal oxygen (HFNO) or continuous positive airways pressure systems if needed. This has required a change in the methods for supply and monitoring of oxygen use within and across hospitals to enable sustained use of this treatment in a high proportion of patients with respiratory failure9.
Children
Initially thought to be relatively unscathed by infection with SARS CoV2, over 1000 cases of a multisystem inflammatory syndrome, known variously as pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) or multisystem inflammatory syndrome in children (MIS-C), has been reported from multiple countries. Affected children present with persistent fever accompanied, to a variable extent, by gastrointestinal symptoms, rash, and conjunctival inflammation10. Affected children are usually older than children presenting with a similar condition, Kawasaki syndrome, and have high circulating concentrations of ferritin, D-dimer and troponin which are rarely seen in Kawasaki disease. Myocarditis, transient myocardial dysfunction, and shock are present in approximately half of UK and U.S. case series and a common cause of death in those affected10. The question as to whether children are a major source of transmission remains open and awaits more detailed studies.
Community Care
Public Health Measures
Given the recognition of the transmissibility of the SARS-Cov-2 virus, strategies which prevent human beings mixing closely together have successfully controlled community spread of infection. However, whilst these strategies control the rise in hospital admissions and the associated death rates, both are economically catastrophic and psychologically damaging, for adults and children10,11,12. As we have seen In the occurrence of a second wave of rising infections, hospital admissions and deaths after Christmas, preventing resurgent disease on returning to greater mixing in schools, factories, offices and shops requires a number of approaches.
Testing for COVID-19
With the early January lock down in the UK cases, hospitalisations and deaths have declined across the country. There is an easily recognised sense of relief among many at the thought of returning to work/school and to ‘normal’ life. However, once public mixing on public transport and in schools, offices and shops returns to higher levels, the potential for increased transmission remains while the virus remains in circulation within the community, albeit at a low level.
Workplaces should develop policies encouraging individuals with any suggestive symptoms not to come to work and to seek testing. However asymptomatic infections account for between 20 and 40% of patients with COVID-19 and they are able transmit the virus to uninfected people13. Viral culture studies also suggest that an infected person can transmit the infection from 2 days before and for up to 7 days after first onset of symptoms14. On return to work a strategy for detecting asymptomatic infection may be helpful to reduce spread from asymptomatic carriers. The UK government has offered rapid antigen testing to workplaces to enable employers to consider regular testing of their workforce. Individuals testing positive can be sent home and transmission within the workplace potentially diminished. This strategy has been pursued using nucleic acid antigen testing (NAAT) and self-administered rapid antigen tests15,16. Self-administered rapid antigen testing is attractive for this approach as it is less expensive than NAAT and gives a result within a timescale practical for use in a workplace setting, however more information is needed on the potential for false positive and false negative results with these assays17. In addition, rapid antigen testing cannot identify the specific variant causing infection in the individual, and secondary NAAT confirmation, which provides an RNA signature which can be used for variant screening, should be considered.
Because of this uncertainty, and the potential for false negative test results17, additional steps to prevent transmission within the workplace remain important. These include remote working where possible, and where not (eg for transport, factory, hospitality, school and shop workers) mask wearing, spacing/shielding between individuals, handwashing, surface cleaning to prevent fomite transmission together with office ventilation strategies which diminish risk of build-up of aerosols are all valuable to prevent interindividual spread. Constant screening of individuals for signs of infection with prompt isolation of individuals becoming unwell at work (as well as close contacts) will also be important.
Vaccination
The rapid development and roll out of multiple successful vaccines has undoubtedly been a highlight of the past year18,19. In the UK vaccination to date has been initially focused on those at highest risk of death from infection, to alleviate pressure on hospital care services and reduce mortality20. However, this strategy permits continued circulation of infection within the unvaccinated lower risk community. Early information from vaccinated cohorts has suggested potential for prevention of infection as well as prevention of disease21. If this is sustained, then extension of vaccination to include younger low risk adults and children would further decrease risk of sustained community spread and enable the use of ‘ring vaccination’ to control localised outbreaks22.
In this circumstance, the increased ‘selection pressure’ exerted by ‘herd immunity’ may, ironically, result in the emergence of ‘escape’ variants – infections which are less susceptible to vaccine induced immune responses – and thus the need to identify infection and prevent contact between infected persons will remain important. Some individuals may not respond to vaccination and thereby represent a continued potential for sustained spread of infection particularly in healthcare settings23. For these individuals, and to be prepared in case of an emergent epidemic of a vaccine resistant variant, antiviral treatments that can be used as an alternative to vaccination for early treatment of disease to prevent need for hospitalisation and to prevent spread, continue to be needed.
Continue development of antiviral medications for early treatment and chemoprophylaxis
The current separation between testing for COVID-19 and access to clinical care has resulted in limited studies of treatment of the virus or prophylaxis in the community. Regrettably, with the possible exception of remdesivir, repurposing of antiviral medications for treatment of COVID has had limited success. Data from studies of remdesivir have been mixed. Although use of the agent successfully reduced time to recovery in one study, a large subsequent international study failed to replicate the results24,25. Gilead is now investigating remdesivir given via inhalation; this route of administration would be less onerous for community use than IV administration twice daily26. A second IV antiviral, the adenosine analog galidesivir, showed some promising dose related antiviral effects in a PI trial in Brazil but no further trials have been started27.
Investigation of convalescent plasma in the UK has been limited to use in hospitalised patients in the RECOVERY program and no effect was observed28. Mean time to treatment in this study was 9 days after first onset of illness. As viral load generally peaks 2-3 days post first onset of symptoms in COVID patients, the lack of effect might have been anticipated in this patient group. In common with other respiratory viral disorders, infection of the respiratory tract triggers an innate immune response in many subjects, which can effectively reduce viral burden within the first week of illness. Treatments intended to reduce viral burden and change disease trajectory should start early and possibly no later than 4-5 days post disease onset29,30,31.
Several pharmaceutical companies have used existing monoclonal antibody (MAb) technology to produce anti SARS CoV2 monoclonal antibodies directed against various viral antigens. Two antibody products, REGN-CoV2 (a combination of casirivimab and imdevimab) and the Eli Lilly product, bamlanivimab, either as monotherapy or in combination with etesevimab, are now available for use in COVID-19 affected patients at high risk of complicated illness in the EU under Article 5(3) procedures. Both of these products have demonstrated rapid viral load reduction and prevention of hospitalizations and deaths when given as a single administration early in the course of disease (4-5 days post onset). Treatment is administered as an infusion over 20-60 minutes (dependent on fluid volume administered). Infusions should be administered in a centre able to deal with potential infusion reactions but these could include an outpatient setting, using trained staff and a travelling infusion ‘bus’ as used in the clinical trial programs32,33. In addition, administration can be completed in care homes and other residential institutions, as required, for outbreak control.
Drugs given via inhalation are currently being investigated in both a hospitalised and outpatient setting26. Inhaled interferon beta has shown preliminary evidence of efficacy in hospitalised patients34 and results of a study in community infection are awaited. Inhaled budesonide started within 7 days of symptom onset (mean 3 days) was also shown to reduce hospitalisation rate and duration of disease in subjects treated in the community. This may be a function of a dual anti-inflammatory and antiviral mechanism of action35. Other inhaled agents being investigated for COVID include remdesivir, hydroxychloroquine, niclosamide and PUL-04226.
A number of oral antiviral medications remain under investigation. Orally active therapies with antiviral effects noted in cell culture and/or in animal studies include the antiprotozoal agent nitazoxanide and the broad-spectrum antiviral agent molnupiravir (EIDD-2801). A recent press release from Ridgeback Biotherapeutics and Merck suggested that administration of molnupiravir, an orally active prodrug of the synthetic nucleoside derivative N4-hydroxycytidine which inhibits viral replication by inducing copy errors, resulted in faster clearance of culturable virus from nose/throat swabs in an early phase trial. In the 32% of subjects with SARS CoV2 culture positive nose/throat swabs at baseline, 0/47 molnupiravir treated patients were still culture positive on Day 5 compared to 6/25 (24%) placebo recipients (p-0.001)36. These data suggest a potent antiviral effect, which merits further investigation for early outpatient treatment to prevent hospitalisation and death from COVID in patients at high risk of adverse outcomes. Similarly, nitazoxanide, while used clinically for the treatment of cryptosporidia/giardia lamblia diarrhoeal disease, has broad spectrum antiviral properties. The mechanism of action of nitzoxanide vs SARS CoV2 is uncertain but may be a result of interference with viral nucleoprotein activity. These products are also being investigated in an AGILE early phase platform trial in the UK37.
All of these approaches offer options for the management of COVID disease in the community with the aim of reducing the need for hospital care and its associated mortality. Effective antiviral treatment has been successfully used prior to availability of and in conjunction with vaccination in pandemic influenza38. This approach provides a model for the future management of COVID.
Looking to the Future
SARS-CoV-2 is not yet under control and a further wave of high hospital admission and deaths is likely, as has progressed in continental Europe. The rate of appearance of new variants remains a concern and is likely to continue while widespread community outbreaks remain unchecked, as has been observed in South Africa and Brazil39,40. To counter these threats we urgently need to step up research on antivirals. Emphasis should focus on drugs suitable for use in the community to reduce epidemic spread in the face of vaccine resistant emergent variants, reduce need for hospitalisation and reduce mortality, as observed previously with anti-influenza antivirals38.
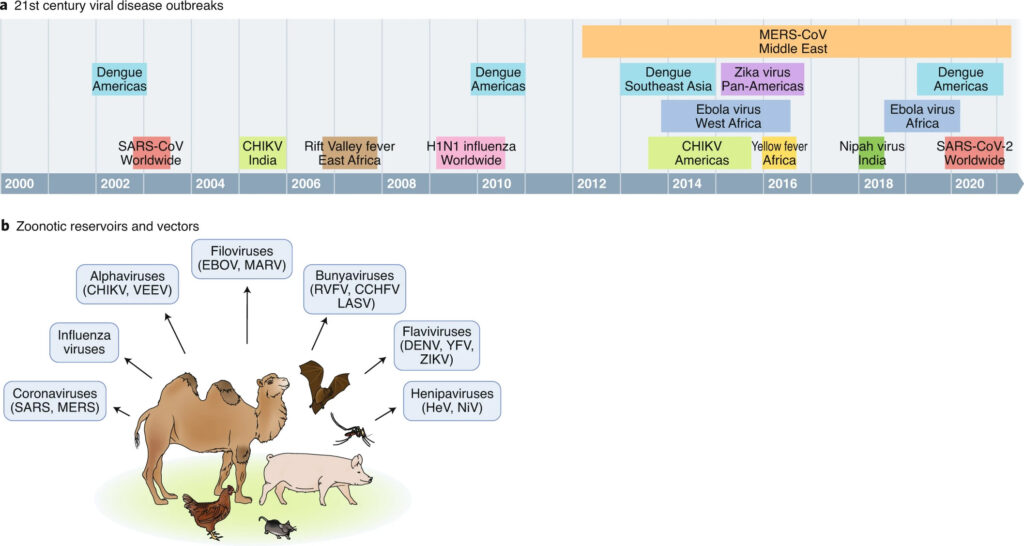
During the first 20 years of this century the world has experienced 10 epidemics of serious viral disease. Many have emerged from zoonotic reservoirs (Figure 3)41. If we are not to experience another pandemic as devastating as this one has been- and without continued investment in research into effective community use of antivirals may continue to be – we must invest in proactive research and development of novel therapeutics to address this, and future, threats.
Conclusions
This year’s ride with the first horseman has been a bruising experience, with over 115 million recorded infections and approaching 3 million deaths worldwide to date. The rapid deployment of novel vaccine strategies has been a particular success, but, despite the extensive efforts made, vaccinating the world will take another year or more to achieve, while continued community spread of disease risks the potential emergence of vaccine escape mutant viruses. New methods of control are needed for this pandemic and for other potential emergent threats. This undertaking requires a new vision, dedicated funding and partnership, but, ultimately, offers hope that global populations need never again experience a pandemic as devastating as COVID-19.
References
- Revelation 6:2
- Siddiqi H K, Mehra M R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020; 39:405-407. doi: 10.1016/j.healun.2020.03.012.
- The RECOVERY Trial Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021; 384:693-704
- https://www.recoverytrial.net/files/recovery-press-release-tocilizumab_final.pdf
- The REMAP-CAP Investigators. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med 2021. DOI: 10.1056/NEJMoa2100433
- Rentsch CT et al., Early initiation of prophylactic anticoagulation for prevention of covid-19 mortality in patients admitted to hospital in the US BJM 2021; 372:n311
- Hunt BJ et al, Risk-benefit balance may depend on illness severity BMJ 2021;372 : n487
- ICNARC report on COVID-19 in critical care: England, Wales and Northern Ireland: 12 March 2021 https://www.icnarc.org/Our-Audit/Audits/Cmp/Report Accessed 15 March 2021
- https://www.iheem.org.uk/wp-content/uploads/2021/01/Oxygen-Consumption-Monitoring-within-The-University-Hospitals-of-Leicest…pdf
- Snape, M D & Viner, R M. COVID-19 in children and young people. Science 2020. doi.org/10.1126/science.abd6165.
- Ceylan RF et al. Historical evidence for economic effects of COVID-19. Eur J Health Econ. 2020 Aug;21(6):817-823. doi: 10.1007/s10198-020-01206-8. PMID: 32500243; PMCID: PMC7270155.
- Heitzman J. Impact of COVID-19 pandemic on mental health. Psychiatr Pol. 2020 Apr 30;54(2):187-198. doi: 10.12740/PP/120373. Epub 2020 Apr 30. PMID: 32772053.
- Rivett L et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020 May 11;9:e58728. doi: 10.7554/eLife.58728. PMID: 32392129; PMCID: PMC7314537.
- Cevik M et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe 2020. doi:10.1016/S2666-5247(20)30172-5.
- Chin ET et.al., Frequency of Routine Testing for Coronavirus Disease 2019 (COVID-19) in High-risk Healthcare Environments to Reduce Outbreaks. Clin Infect Dis. 2020 Oct 26:ciaa1383. doi: 10.1093/cid/ciaa1383. Epub ahead of print. PMID: 33570097; PMCID: PMC7797732.
- Downs LO et al. Home-based SARS-CoV-2 lateral flow antigen testing in hospital workers. J Infect 2021; 82: p282-327.
- Wise J. Covid-19: Lateral flow tests miss over half of cases, Liverpool pilot data show BMJ 2020; 371 :m4848 doi:10.1136/bmj.m4848
- MHRA Public Assessment Report Authorisation for Temporary Supply COVID-19 mRNA Vaccine BNT162b2 (BNT162b2 RNA)concentrate for solution for injection. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/944544/COVID-19_mRNA_Vaccine_BNT162b2__UKPAR___PFIZER_BIONTECH__15Dec2020.pdf
- MHRA Public Assessment Report Authorisation for Temporary Supply COVID-19 Vaccine AstraZeneca, solution for injection in multidose container COVID-19 Vaccine (ChAdOx1-S [recombinant]). https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/963928/UKPAR_COVID_19_Vaccine_AstraZeneca_23.02.2021.pdf
- Joint Committee on Vaccination and Immunisation: advice on priority groups for COVID-19 vaccination, 30 December 2020. https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020/joint-committee-on-vaccination-and-immunisation-advice-on-priority-groups-for-covid-19-vaccination-30-december-2020
- Bernal JL et al. Early effectiveness of COVID-19 vaccination with BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on symptomatic disease, hospitalisations and mortality in older adults in England. doi: https://doi.org/10.1101/2021.03.01.21252652
- MacIntyre C Raina et al. Modelling of COVID-19 vaccination strategies and herd immunity, in scenarios of limited and full vaccine supply in NSW, Australia. doi: https://doi.org/10.1101/2020.12.15.20248278
- Monin-Aldama L et al. Interim results of the safety and immune-efficacy of 1 versus 2 doses of COVID-19 vaccine BNT162b2 for cancer patients in the context of the UK vaccine priority guidelines. https://www.immunophenotype.org/index.php/sample-page/vaccination-of-cancer-patients/immune-efficacy-of-bnt162b2-vaccine/
- Beigel JH et al. Remdesivir for the Treatment of Covid-19 — Final Report. N Engl J Med 2020; 383:1813-1826
- WHO Solidarity Trial Consortium. Repurposed antiviral drugs for COVID-19 –interim WHO SOLIDARITY trial results. N Engl J Med 2021; 384:497-511
- Humphries, B. et al (2020), ‘Inhalation therapies for COVID-19’, Faculty of Pharmaceutical Medicine blog, 28 January 2021. Available at: https://www.fpm.org.uk/blog/inhalation-therapies-for-covid-19/ (Accessed: 11 March 2021).
- https://finance.yahoo.com/news/biocryst-provides-galidesivir-program-120000612.html
- Horby P et al. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. doi: https://doi.org/10.1101/2021.03.09.21252736
- Pan Y et al. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis 2020 https://doi.org/10.1016/S1473-3099(20)30113-4
- Zou L et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020; (published online Jan 30.) DOI:10.1056/NEJMc2001737
- Liu Y et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 2020. DOI:https://doi.org/10.1016/S1473-3099(20)30232-2
- https://www.ema.europa.eu/en/news/ema-issues-advice-use-regn-cov2-antibody-combination-casirivimab-imdevimab
- https://www.ema.europa.eu/en/news/ema-issues-advice-use-antibody-combination-bamlanivimab-etesevimab
- Monk PD et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med 2021; 9: 196–206.
- Ramakrishnan S, Nikolau DV et al. Inhaled budesonide in the treatment of early COVID-19 illness: a randomised controlled trial. https://www.medrxiv.org/content/10.1101/2021.02.04.21251134v1
- https://www.merck.com/news/ridgeback-biotherapeutics-and-merck-announce-preliminary-findings-from-a-phase-2a-trial-of-investigational-covid-19-therapeutic-molnupiravir/
- https://www.southampton.ac.uk/ctu/news/2021/02/15-government-funding-boost-for-covid-19-drug-testing-platform.page?
- Muthuri SG et al. Impact of Neuraminidase Inhibitor Treatment on Outcomes of Public Health Importance During the 2009–2010 Influenza A(H1N1) Pandemic: A Systematic Review and Meta-Analysis in Hospitalized Patients. J Infect Dis 2013; 207: 553–63
- Tegally, H., Wilkinson, E., Giovanetti, M. et al. Emergence of a SARS-CoV-2 variant of concern with mutations in spike glycoprotein. Nature (2021). https://doi.org/10.1038/s41586-021-03402-9
- Faria NR et al. Genomics and epidemiology of a novel SARS-CoV-2 lineage in Manaus,Brazil http://dx.doi.org/10.1101/2021.02.26.21252554.
- Meganck, R.M., Baric, R.S. Developing therapeutic approaches for twenty-first-century emerging infectious viral diseases. Nat Med 2021; 27: 401–410. https://doi.org/10.1038/s41591-021-01282-0
Read more from Prof Penny Ward

How can we deploy the available toolkit to reduce the burden of respiratory viral infections on the NHS in 2022-3?
New blog by Penny Ward

Professor Penny Ward wins FPM Volunteer Award
From July 2022
Professor Ward won our brand new award for her services to FPM

It’s All Greek to Me: SARS-CoV-2 Variants, Vaccines and Antivirals
A Deep Dive by Professor Penny Ward et al
A Year with the ‘First Horseman’
Penny Ward reflects on what we have learned one year after the WHO announced the start of the COVID-19 pandemic.

Pregnancy, COVID-19 and Emerging Therapeutic Options
Allyah Abbas-Hanif, Sue Tansey, Sankarasubramanian Rajaram, Penny Ward

The End of the Transition Period and Recent announcements from the MHRA
What do you need to know for 1 January 2021?