FPM Annual Report and Accounts
For the year ending 31 December 2022
Forewords
From the President and the Chief Executive

My first full year in office started while we were still in lockdown, then moving into a difficult time for the NHS backlog and a financially challenging economy with an increase in inflation in UK to 11.1% in October 2022 and recession only missed by 0.1%!
Nonetheless, the visibility of pharmaceutical medicine continues to rise. The recognition, after COVID, of how important pharma and the life science sector is to the UK has put many pharmaceutical physicians into the spotlight nationally and globally. The two sectors in which the majority of FPM membership work, are the non-manufacturing parts of biopharma and its service sectors (employing some 85,000 people)[1]. It is estimated there are over 4500 physicians in these sectors in UK alone, often fulfilling critical leadership roles. This is further acknowledged by FPM’s extensive programme of meetings, on many topics, with national organisations of the 4 nations, AMS, AoMRC, GMC, NHSE, NIHR, DHSC, BPS, OLS and many more[2]. The emergence of COVID treatments led the Chief Medical Officer England inviting us to summarise them and FPM was prompted by the Chief Scientific Adviser to run a sponsored multidisciplinary workshop across pharma, academia, government, regulatory and patient associations (DEMENDE[3]). The resulting report made recommendations for change to COVID research, regulatory assessment and access.
In a busy and varied year for policy work, FPM also contributed to an extensive piece of work on sepsis for AoMRC which is now being adopted into NICE guidance. The Rare Disease Expert Group ran two webinars and collaborated with AMS on a workshop on Clinical Trials for Rare and Ultra-rare diseases. FPM gave evidence to the Birmingham Health Partners Report on The Healthy Mum, Healthy Baby, Healthy Future: The Case for UK Leadership review and we were co-authors of the Menopause Practice Standards with the British Menopause Society. Clinical trials and devices MHRA consultations were also supported, as was the GMC Good Medical Practice Review consultation and EDI projects and the CPSA[4] initiative with BPS. Medical undergraduate training in pharmaceutical medicine is also continuing and expanding.
The journal clubs, fireside chats, webinars and the Annual Symposium and Education Day offer members valuable updates. Broadened membership criteria recognising some international qualifications as equivalent to DPM has been introduced. FPM Global has explored initiatives to provide education and examinations internationally (initially focussing on the USA, Australasia, Middle East and Africa). The Board of Examiners have maintained the online examinations and the online DPM training and PMST is migrating to the new curriculum with new assessment days for potential trainees.
Financially, FPM has been hit hard like all colleges by the impacts of the economy on investments, rising costs and a challenging trading environment. Remedial actions are being taken and FPM will be embarking on a fundraising campaign to redress the impact on our assets and allow us to further develop our charitable impacts. It is exciting that Pharmaceutical Medicine is recognised as an important contributor to public health as part of the leadership of the key Life Sciences Vision[5] and recent announcements in the 2023 Budget and the recent Pioneer report[6] provide us enormous opportunity. Our FPM 2023-2025 strategy visualises substantial growth for FPM in influence and membership and we look forward to exciting times ahead.
[1] Bioscience and health technology sector statistics 2020 – Government Website
[2] AMS = Academy of Medical Sciences, AoMRC = Academy of Medical Royal Colleges, BPS = British Pharmacological Society, GMC = UK General Medical Council, NHSE = National Health Service England, DHSC = Department of Health and Social Care, NIHR = National Institute of Health and Care Research, NICE = National Institute of Health and Care Excellence, OLS = Office of Life Sciences
[3] DEMENDE = DEfining MEdical NeeDs and Evidence
[4] CPSA = Clinical Pharmacology Skills Alliance
[5] Life Sciences Vision Policy Paper – Government Website
[6] Pioneer: global science for global good – Government Website
FPM’s highlights in 2022
Board of Trustees' Report
The trustees are pleased to present their annual report together with the audited financial statements for the financial year ended 31 December 2022. The financial statements comply with current statutory requirements, the Memorandum and Articles of Association and the Statement of Recommended Practice – Accounting and Reporting by Charities (SORP FRS 102).
Our purpose
To advance the science and practice of pharmaceutical medicine by working to develop and maintain competence, ethics and integrity and the highest professional standards in the specialty for the benefit of the public.
Public benefit
The charitable purposes of The Faculty of Pharmaceutical Medicine (FPM) are set out in the Memorandum and Articles of Association and are:
- to promote the science of pharmaceutical medicine.
- to develop and maintain competence, ethical integrity and high professional standards in the practice of pharmaceutical medicine; and
- to advance knowledge in pharmaceutical medicine.
Pharmaceutical medicine is the medical specialty concerned with the discovery, development, evaluation, licensing and monitoring of medicines and the medical aspects of their marketing.
FPM seeks through its activities to bring about an improvement in the health of the public and patients. Our activities seek to advance the science and practice of pharmaceutical medicine by contributing to the provision of effective medicines for public benefit. The trustees regularly review the aims, objectives and activities of the charity referring to the Charity Commission’s guidance on public benefit.
Our vision
A world where effective medicines meet the needs of patients.
Our mission
To advance the science and practice of pharmaceutical medicine for the benefit of the public.
We will do this through four strategic priorities:
- Set and appraise standards for training in and practice of pharmaceutical medicine
- Promote understanding of pharmaceutical medicine to create trust
- Engage with clinical doctors to promote pharmaceutical medicine as a career option and support all FPM members in their training and practice
- Ensure good governance and financial stability

Our values
How we deliver the strategic objectives will be guided by the following values that will guide staff and members’ behaviour:
| We Are: | This Means: |
|---|---|
| Professional | Being accountable for our work and actions |
| Innovative | Seeking solutions proactively |
| Caring | Treating everyone with dignity |
| Collaborative | Working positively with others |
| Credible | Being honest and ethical in our work |
| Learned | Investing in developing knowledge and skills |
FPM welcomed Dr Kamlesh Sheth to the Board of Trustees as the new Treasurer and Dr Ado Muhammad as a Fellow Trustee and first Nigerian to serve on the Board.
FPM Strategy 2020-2022
2022 was the final year of Strategy 2020-2022 and delivery against the objectives continued to be dominated by the response to the COVID-19 pandemic, principally the DEfining MEdical Needs and eviDEnce’(DEMENDE) project.
The four strategic objectives of the strategy were:
- Setting standards for training in pharmaceutical medicine
- Promoting pharmaceutical medicine to create trust in the specialty nationally and internationally
- Supporting and engaging members in their practice
- Develop FPM as a sustainable and influential organisation
So what did FPM achieve in 2022?
Objective 1: Setting standards for training in pharmaceutical medicine
The Office of the Board of Examiners oversees the standards and delivery of examinations administered by FPM.
In 2022 it was agreed to open up the Certificate of Pharmaceutical Medicine (CPM) examination to non-physicians to ensure that all those working in pharmaceutical medicine have the opportunity to evidence the standards to which they work. The Diploma in Pharmaceutical Medicine (DPM) remains an examination that only physicians will be able to undertake. The examinations were successfully delivered with most candidates participating remotely via the TestReach system and with three candidates opting to sit the papers in the FPM office.
Diploma/Certificate in Pharmaceutical Medicine
Diploma/Certificate in Human Pharmacology
- 7 candidates sat the CHP exam
- 2 candidates sat the DHP exam (paper 2)
- 3 candidates sat the DHP exam (paper 3)
- 2 candidates were awarded DHP after submitting their training portfolios for review
Pharmaceutical Medicine Specialty Training (PMST)
- 21 candidates enrolled on the PMST programme
- 18 candidates completed the programme
- 12 pharmaceutical physicians were entered in the GMC’s specialist register
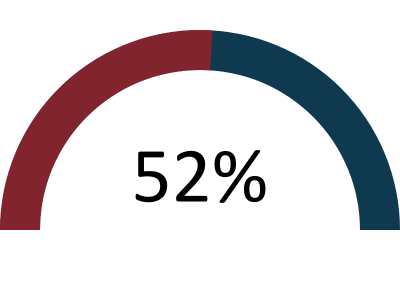
The GMC’s annual national training survey went live between 22 March 2022 and 17 May 2022 with 82% of FPM trainees and 70% of ESs completing the survey. The overall satisfaction of trainees and ESs was 80% and 72.5% respectively.
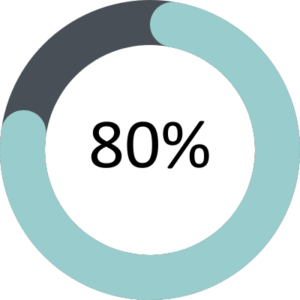
Trainees
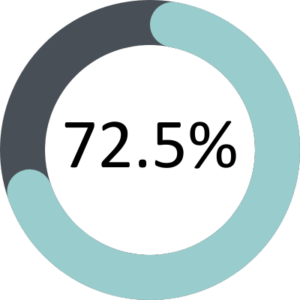
Educational Supervisors
Objective 2: Promoting pharmaceutical medicine to create trust in the specialty nationally and internationally
FPM continues to raise awareness of and advocate publicly for the science and practice of pharmaceutical medicine and the benefits that the specialty can bring to the health of global populations.
Policy and communications
During 2022 we have continued to support a public understanding of the COVID-19 pandemic, with a particular focus on antiviral treatments and their deployment. In order to harness the expertise of our members, along with other stakeholder groups, FPM developed the COVID-19 ‘DEfining MEdical Needs and eviDEnce’ (DEMENDE) multidisciplinary workshops. The outputs from the workshops have suggested approaches to encourage, incentivise and innovate in R&D and optimise the use of medical interventions in COVID-19 healthcare.
To support objectives to promote pharmaceutical medicine, a series of three webinars were delivered in conjunction with the ABPI to help medical directors and other leaders in embedding the ABPI Principles within their organisations. The sessions focused on: an Introduction to the ABPI Principles; the ‘Patients’ principle; and the Integrity, Transparency and Respect principles.
The Policy and Communications Group and associated expert groups have also been busy throughout the year responding to a number of important public consultations as follows:
FPM Global
FPM Global was launched as a new committee in 2021 to provide leadership in establishing pharmaceutical medicine as a global medical specialty and to increase FPM’s global reach through the creation of a network of pharmaceutical physicians who work outside of the UK. In 2022, under the direction of the Chair, Dr Viraj Rajadhyaksha, plans were scoped and agreed to focus on the following:
- Build the web page, create a social media campaign and raise the profile of FPM Global and its members
- Start the process to develop workstreams and networks in regional areas
- Consult with external bodies such as RCP London to learn from their experience
The work of FPM Global is still in the embryonic stage but there have been some notable successes. In June, the President, Dr Flic Gabbay, attended a meeting with FPM members and non-members in San Diego, USA where they reviewed the current offering to overseas based pharmaceutical physicians, especially the lack of training and educational development opportunities for new entrants as well as current members. In October, Professor Peter Stonier presented a session entitled “From training to trusting” at IFAPP’s International Conference on Pharmaceutical Medicine 2022 with PMST Trainee, Dr Jaya Chidambaram who spoke about her experience of the PMST training programme.
In November, Dr Penny Ward presented at the Australian Association of Pharmaceutical Industry Professionals and the Medical Affairs Professional Society joint workshop which was organised by Dr Victoria Elegant. Dr Ward presented a summary of the educational provision of FPM.
The President accepted an invitation to present on the Pharmasteer programme in December. The programme is co-organised by a medical school in India and an industry sponsor with the aim of introducing medical graduates in India to practical topics relating to medical affairs.
FPM Designated Body
FPM is a Designated Body for providing annual appraisals and GMC revalidation. On 31 December 2022, 668 members had a prescribed connection to FPM as a designated body, the highest number of connected doctors since the introduction of revalidation at the end of 2012.
There has continued to be a greater focus on health and wellbeing at the appraisals during the pandemic and, with NHS England (NHSE) and GMC backing, there continues to be considerable flexibility for doctors whose lives have been impacted either personally or professionally by the pandemic. This rebalancing and support has been appreciated by our doctors. NHSE guidance continues to allow appraisals to take place via video conference. In order to understand the impact of video appraisals we undertook a survey with our appraisers, and it showed there was almost unanimous support by appraisers for consideration of appraisals by videoconference to be permitted for at least some of the appraisals in each cycle. The results provided strong reassurance that the quality and benefit of appraisals has not been compromised by the need to undertake appraisals remotely during the pandemic.
During the 2022/23 appraisal year, 111 doctors connected to the FPM designated body and 72 disconnected. Those doctors coming to us from the NHS join us with first-hand experience of the huge challenges the NHS has faced during the pandemic – and continues to face. A number of our doctors have continued to support the covid vaccination program.
We were very pleased to welcome Dr Liz Clark to join the Appraisal Lead team alongside Dr Asad Khan and Dr Mike Perkins, as Dr Sharon McCullough stepped down during the year to take on the role of Director of Training and Development at FPM. Quarterly meetings with the Appraisal Leads continue to be valuable for ensuring consistency and generating quality improvement ideas. For example, there has been a collaborative effort in producing new online guidance for completing the PReP Input Form, which we hope will help to make the process more straightforward. The new guidance, ‘How To Complete Your Input Form’, can be viewed here. The Appraisal Leads also deliver an online introduction to appraisal and revalidation every six weeks. This is mainly for newly connecting doctors, but it is open to all connected doctors, in case they need a refresher. We are fortunate to have 79 highly enthusiastic appraisers. Of these, we have 20 appraisers who are connected to other designated bodies including, for example, in the NHS. This helps to ensure similar standards across pharma and benchmark with other designed bodies. We have greatly valued the advice and input we have received from our Lay Advisor William Payne throughout the year.
Objective 3: Supporting and engaging members in their practice
The DPM Training Programme was delivered successfully for the third year. The programme is being converted to a new digital format that will be delivered from FPM’s new learning management system (LMS), the FPM Learning Hub, from 2023.
Professor Penny Ward and Dr Sharon McCullough collaborated with the Royal College of Physicians to develop the content for an eLearning module titled, “Working with Investigational Medicines”, which is one of a series of courses that aims to develop clinical research skills in medical professionals on behalf of NIHR.
Professor Ward and Dr Jeymi Tambiah co-created and delivered FPM’s first bespoke in-company Physicians Onboarding Development programme for a pharmaceutical company based in the US. The successful programme was delivered twice and has been recommissioned for 2023.
In addition, the first in a new series of Masterclasses, The Fundamentals of Patient Engagement for Pharmaceutical Physicians was launched and followed on from a successful panel discussion on how to embed patient engagement in practice for pharmaceutical physicians.
Objective 4: Develop FPM as a sustainable and influential organisation
To ensure its future sustainability, FPM is investing in its digital infrastructure which will enable it to streamline its processes, increase its efficiency and deliver a more modernised approach to its members to foster greater engagement.
Currently, FPM is delivering two major change projects: the Customer Relationship Management system (CRM) and the Learning Management System (LMS) which will both be completed in 2023. FPM would like to thank the Dinwoodie Charitable Trust for its generous donation towards the cost of implementing both the CRM and the LMS.
As at 31 December 2022 FPM has 1573 FPM members in 39 countries with 80% based within the United Kingdom and 20% based in other countries. The members of FPM are its lifeblood and attention has been focused on increasing the membership across all categories. Membership has grown by 2.3%, the second year of growth following a slow decline since 2014. Our strategy to increase the number of Members (MFPM) has been successful, leading to growth in the number of MFPM for the first time since 2013.
There is also significant growth in the Affiliate membership, but the number of Fellows has declined and reversing this trend will be a focus for 2023. The retention rate has remained at 95.5% which is excellent compared to industry standards.
The equality, diversity and inclusion (EDI) agenda, led by Dr John Ndikum, continues to be a cross-cutting theme across all functions and receives high engagement from the membership where EDI members volunteer on event, policy and project working groups to ensure outputs are accessible to the spectrum of the membership. EDI Forum members have contributed to various FPM consultation responses, including the submissions for the GMC’s Good Medical Practice consultation and the UK government’s Equity in Medical Devices consultation.
The EDI Forum delivered several online events this year including a webinar with the Yale School of Public Health about their pioneering EDI initiatives, as well as several Fireside chats – with Dr Marc Watson for World Mental Health Day 2022 and with Lamont Terrell for Black History Month 2022. Multiple blogs were published by the EDI Forum including a piece about ‘Diversity in Action’ about working in a diverse organisation, authored by Forum member Dr Ansuya Naidoo for the World Day for Cultural Diversity for Dialogue and Development. FPM marked several observances for the first time with written pieces; Dr John Ndikum penned an article about the Windrush Generation for Windrush Day 2022, and FPM interviewed a number of FPM Fellows for Pride and South Asian Heritage Month.
FPM launched the Women in Pharmaceutical Medicine report, which evaluated potential differences in the experience and barriers in the career progression of women in pharmaceutical medicine and we thank the Royal Society of Chemistry for funding this important work. A specific workstream to explore the experiences of black women was also developed to address the intersectionality of race and gender.
FPM is a member of the Inequalities in Health Alliance, which is led by RCP London, and continues to contribute to its advocacy work. FPM also became a member of the UK Health Alliance on Climate Change.
An important project on making membership accessible to those working as pharmaceutical physicians was led by the Routes to Membership Working Group which reviewed the criteria for the award of Membership (MFPM) and resulted in the widening of the number of qualifications which will be accepted for the award of MFPM.
FPM’s Strategy 2020-2022 was delivered against the turmoil caused by the COVID-19 pandemic, but it helped to spearhead FPM’s modernisation to expand the number of services and products it offers. This evolution and modernisation continues with our new Strategy 2023-2025.
Engaging our members and stakeholders
FPM’s holds both free and paid-for events throughout the year. The popularity of our online events means we will continue to run them alongside our face-to-face activities. Our aim is to deliver a varied programme of events which are accessible and relevant to all our members, as well as to interested non-members. 2022’s Annual Symposium was our first fully hybrid event, running both in person in London and online.
We wanted to make sure our online delegates had an equivalent experience to those attending in-person so we invested in a fully hosted online platform that allowed our delegates to network, move between sessions and ask questions.
Going forward we expect to run more hybrid events as they give our members the opportunity to meet in person or the convenience of attending online. Many of the events are eligible for CPD and are available on FPM On Demand for members and non-members to catch up at their convenience.
A summary of the events undertaken by FPM in 2022 is provided below:
| Events | Training | Free to attend events |
|---|---|---|
| FPM Annual Symposium 2022 (hybrid): Can we? Should we? – Fostering trust through ethical practice | Preparing for the Diploma in Pharmaceutical Medicine (DPM) Exam: online (free) | Fireside chat with Dr Bu Siakpere: improving EDI in our day-to-day practice: online |
| FPM Education Day 2022 (in person): The Real Deal - The use of real world evidence in pharmaceutical medicine | DPM Training Programme: online | Fireside Chat with Marc Watson: From surviving to thriving with my mental health: online |
| Advancing the Frontiers of Gene Therapy and Rare Diseases: online | Managing Medical Emergencies in Human Pharmacology: online | Fireside Chat with Lamont Terrell: From Medicinal Chemist to Diversity Champion: online |
| The Code in a Day: a guided tour of the ABPI Code of Practice: online | EDI in Focus: Yale School of Public Health on leading the way in Diversity, Equity, Inclusion and Belonging: online |
FPM’s virtual Journal Club continues to be popular with members. Papers debated at Journal Club for this reporting period:
- Molecular Landscape and Actionable Alterations in a Genomically Guided Cancer Clinical Trial: National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH) (led by Dr Adam Hexter in May 2022)
- Diagnostics and Treatments of COVID-19: A Living Systematic Review of Economic Evaluations (led by Dr Caz Canavan in June 2022) Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance (led by Dr Anuradha Kulasekaran in July 22)
- Pharmaceutical physician’s role in women’s healthcare and menopause therapy (led by Daniela Hriston September 2022)
FPM Annual Symposium Can we? Should we? – Fostering trust through ethical practice
Our November Symposium is our flagship event and an important opportunity for our members to feel connected to their professional body and to each other. This year we explored how pharmaceutical physicians can continue to improve trust in the industry and medicines, across diverse communities, governments and the life-sciences ecosystem, by embedding ethical practice in all that we do. We were delighted to hear important insights from high profile speakers including Professor Sir Chris Whitty, Professor Ben Goldacre, Dr Ian Hudson, Dr Lode Dewulf, Prof Raanan Gillon and Trishna Bharadia.
Annual Symposium delegate feedback
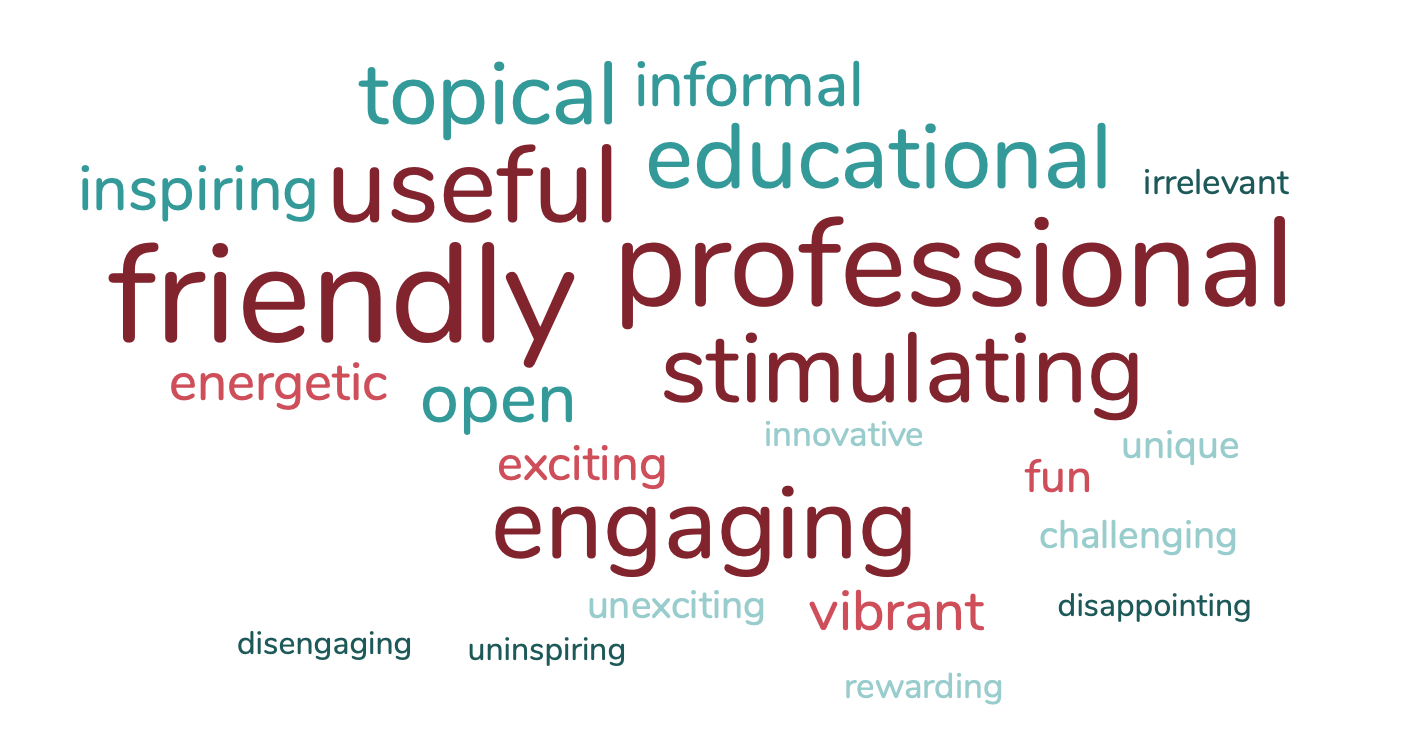
When surveyed shortly after the event, 95.35% of in-person delegates said FPM Annual Symposium 2022 was ‘good’ or ‘very good’ value for money, and when prompted to describe their experience of the event they picked out the following words from a list (larger words were more popular).
We also asked delegates to give additional feedback and below are just a few of the responses, which we share with the responders’ consent. These comments were typical of the glowing feedback we received both via survey and informally.
FPM communications
FPM’s communications continued to thrive in 2022, with an increase in the number of blogs, covering a range of topics. These are supported by regular email communications, including our monthly bulletin, via Mailchimp. These keep members informed and up to date and retain very high levels of engagement vs. industry standards.
FPM continues to engage an expanding audience via social media, delivering thought leadership, links to articles, and posts of FPM statements, event notices and other activities. Our website continues to be a vital source of information to the pharmaceutical medicine community, reaching over 100 thousand annual sessions for a third consecutive year. This is more than double the c.50,000 annual sessions we typically saw with our previous website that was retired in early 2020 and demonstrates a sustained ROI for the current site.
The FPM LinkedIn page remains a key tool in our digital communications. This audience has been developed organically and the most popular posts have been ones which have celebrated the achievements of our members, e.g. during the Annual Awards in June. Our Twitter presence is also being amplified by increasing support from key stakeholders.

FPM Celebrates
2022 marked the 20th year that pharmaceutical medicine was recognised as a UK medical specialty and today as a global discipline, there is increasing demand for pharmaceutical physicians to work or travel abroad.
In 2022, the FPM President’s medal was awarded to Professor Alan Boyd. Professor Boyd has been associated with the FPM since its inception in 1989, and as a former President, he has been an ardent advocate of ensuring that the standards for pharmaceutical medicine are equivalent to those of any other clinical specialty. In 2021, Professor Boyd was awarded Fellowship at the Academy of Medical Sciences for his contributions to medical science and medicine. In 2021, Professor Boyd gifted FPM with the BaxterBoyd DPM training programme and this enduring legacy, his commitment and passion towards educating the next generation of pharmaceutical physicians are duly recognised with the award of President’s medal.
We celebrated the award of Fellowship to the Academy of Medical Sciences for Professor Peter Stonier and Dr Steve Lockhart. FPM’s President, Dr Flic Gabbay was also made an Honorary Fellow of the British Pharmacological Society. New awards were launched in 2022 to recognise academic achievement and excellence, and commitment to FPM and pharmaceutical medicine.
It was another successful year for industry awards for FPM, which was shortlisted in several categories. We thank our staff and volunteers who have contributed to FPM in various capacities and received the deserved recognition from their peers.
The Chief Executive, Dr Marcia Philbin picked up the Highly Commended award in the hotly contested CEO Leadership category at the 2022 Memcom Excellence Awards. FPM was shortlisted also for the Best Integrated Marketing Campaign for PMST.
In the Association Excellence Awards, FPM was shortlisted in three categories: Best Association Newsletter, Blog, Online or Physical Publication (circulation up to 6,500), Association Leadership Award and the Best Longstanding Event, in which FPM won the silver award.
Future Plans
FPM has launched its strategy for 2023-2025, where the focus will be on three pillars of Trust, Sustainability and Relevance.
FPM will launch its new learning management system called FPM Learning and unleash the newly revamped DPM Training programme as eLearning modules. The new CRM will be launched in 2023 and will support FPM as it modernises its processes to deliver greater efficiency and value to its members.
If three change programmes were not enough, FPM will also focus its efforts on growing its membership, especially in the Member and Fellow categories, as it is important it strengthens its pipeline of members. FPM Global will continue to emerge as an important workstream and the EDI Forum will continue to support the delivery of important initiatives, especially the fair training initiative with the GMC. We look forward to the launch of our new podcast and hearing from inspiring pharmaceutical physicians on a range of topics.
An important activity will be to relaunch the FPM values so that we bring to them life and show we care about collaborating to innovate in how we deliver learning to train credible professionals in pharmaceutical medicine.
It is an exciting time to be a pharmaceutical physician so come and join us on our journey!
Thank you
Finally, once again FPM would like to extend thanks to all our members who contributed to our activities in 2022, whether as committee members, examiners, specialty advisers, educational supervisors, appraisers or by supporting raising awareness and advocacy events and policy projects. We truly value your participation and support.