Delivering Impact and Sustainable Growth
FPM Annual Report and Accounts for the year ending 31 December 2024

Forewords
From the President and the Chief Executive

It is a pleasure to introduce the Faculty of Pharmaceutical Medicine’s 2024 Annual Report.
FPM’s mission is to advance the science and practice of pharmaceutical medicine by working to develop and maintain competence, ethics and integrity and the highest professional standards in the specialty for the benefit of the public.
FPM’s commitment to supporting its members remained front and centre with the introduction of FPM Connect, a mentoring and peer-support platform, which fostered meaningful professional relationships across geographies and career stages. We also continued to strengthen our core education offerings, with the DPM Training Programme reaching record levels of participation and recognition, and the launch of the Managing Respiratory Diseases eLearning course helping to expand our contribution to public health education.
Work to expand access to membership and professional recognition came to fruition with the successful launch of a new route to Fellowship. This development was the first piece in a wider membership project that will ensure that FPM can reflect the diversity and expertise of physicians across the pharmaceutical medicine workforce globally.
Our voice in policy and public engagement also grew stronger. Through timely consultation responses, expert-led blogs, and high-impact roundtables on issues like sustainability and diversity in clinical trials, we continued to demonstrate our commitment to thought leadership and collaborative progress across the life sciences. Our growing impact and relevance was perhaps best demonstrated at the 2024 edition of the FPM Annual Symposium, which was our biggest to date.
Internationally, FPM’s influence grew through member-led content and participation in global events. These efforts helped position FPM as a global hub for pharmaceutical physicians and professionals, all united by a shared purpose – to advance health through innovations in medicines, vaccines, medical devices, precision therapies, diagnostics, and digital health technologies.
None of these achievements would have been possible without the dedication of our staff, volunteers, partners, and members. Their expertise, creativity, and shared commitment to excellence are what make FPM thrive.
As we look ahead to the rest of 2025 and beyond, I’m excited to build on this strong foundation. We will continue working to grow our impact, support our members, and shape an increasingly inclusive, innovative and globally connected future for pharmaceutical medicine.

Board of Trustees’ Report
The trustees are pleased to present their annual report together with the audited financial statements for the financial year ended 31 December 2024. The financial statements comply with current statutory requirements, the Memorandum and Articles of Association and the Statement of Recommended Practice – Accounting and Reporting by Charities (SORP FRS 102).
Our purpose
We advance the science and practice of pharmaceutical medicine by working to develop and maintain competence, ethics and integrity and the highest professional standards in the specialty for the benefit of the public.
Public benefit
The charitable purposes of Faculty of Pharmaceutical Medicine (FPM) are set out in the Memorandum and Articles of Association and are:
- to promote the science of pharmaceutical medicine.
- to develop and maintain competence, ethical integrity and high professional standards in the practice of pharmaceutical medicine; and
- to advance knowledge in pharmaceutical medicine.
Pharmaceutical medicine is the medical specialty concerned with the discovery, development, evaluation, licensing and monitoring of medicines and the medical aspects of their marketing. FPM is the home of physicians who advance health through innovations in medicines, vaccines, medical devices, precision therapies, diagnostics, and digital health technologies.
Through its work FPM seeks to bring about an improvement in the health of the public and patients. Our activities advance the science and practice of pharmaceutical medicine by contributing to the provision of effective medicines for public benefit. The trustees regularly review the aims, objectives and activities of the charity referring to the Charity Commission’s guidance on public benefit.
Our vision
A world where effective medicines meet the needs of patients.
Our mission
To advance the science and practice of pharmaceutical medicine for the benefit of the public.
We will do this through three strategic pillars:
- Trust: FPM will be trustworthy by facilitating an increased understanding of the discovery, development and delivery of new medicines, vaccines and medical devices and the interface with public health.
- Sustainability: FPM will be sustainable by building an effective foundation to support our work.
- Relevance: FPM will be relevant to its membership and embrace the wider professional community in pharmaceutical medicine.
The Membership
As of 31 December 2024, there were 1580 FPM members in 38 countries with approximately 80% based within the United Kingdom and 20% in other countries. This year we have seen a 0.9% decrease in the number of members compared to 2023.
The Board of Trustees
There are 14 trustees in total, including two lay trustees and three nominated by each of our three parent medical royal colleges. The remaining trustees are elected, co-opted or appointed from the FPM membership. The Board of Trustees is responsible for the governance of FPM, including setting its overall strategic direction and monitoring progress.
Comittees
The work of FPM is supported by the following committees:
- Finance Committee
- Executive Committee
- Remuneration Committee
- Membership Committee
- Education and Standards Committee
- Fellowship and Awards Committee
- Policy and Communications Group
- Trainees Committee
- FPM Global
- Equality, Diversity and Inclusion Forum
Chairs of the above committees, together with the four Officers of FPM (President, Vice-President, Treasurer and Registrar) as well as senior staff, are members of the Executive Committee.
FPM’s values
How we deliver the strategy is guided by the values that underpin staff and members’ behaviour.
| We Are: | This Means: |
|---|---|
| Professional | This means being accountable for our work and actions |
| Innovative | This means we seek solutions proactively |
| Caring | This means we treat everyone with dignity |
| Collaborative | This means we work positively with others |
| Credible | This means we are honest and ethical in our work |
| Learned | Investing in developing knowledge and skills |
FPM Strategy 2023-2025
Introduction
2024 marked the second year of our strategic plan for 2023–2025, which is built on three key pillars: Trust, Sustainability, and Relevance and examples of what was achieved in 2024 is given below:
| Pillar | Strategic objective | KPI | Action taken |
|---|---|---|---|
| Trust | Setting standards in the discipline through developing education and training programmes, courses and qualifications that build skills and knowledge across the spectrum of pharmaceutical medicine | Deliver small programme (six) of classroom format training online | Delivered “In a day” courses: Code in a Day, and Pharmaceutical Medicine in a Day |
| Sustainability | Exploiting digital technologies through optimising FPM’s digital infrastructure to maximise efficiency and effectiveness in our operations | The finance systems and processes are modernised to achieve costs savings in areas such as credit card fees | New finance tools and processes implemented to modernise budgeting and finance management |
| Relevance | Developing the speciality of pharmaceutical medicine worldwide through facilitating the development of national and global networks of pharmaceutical professionals | Deliver three in company sessions in 2024 | Three in company sessions were delivered |
Over the past year, we remained focused on strengthening both financial and operational resilience by advancing core activities and initiatives aligned with this strategy.
A significant emphasis was placed on enhancing digital infrastructure to improve efficiency and effectiveness. The introduction of a new financial accounting system modernised financial processes, enabling real-time insights into performance.
Another ongoing objective is to deliver new routes to membership, whilst ensuring the maintenance of professional standards. The first part of the work came to fruition in 2024 when a new route to Fellowship was launched, yielding a large cohort of new Fellows of FPM.
Supporting our members also remains a priority. The launch of FPM Connect in May 2024 has facilitated meaningful professional connections by linking members seeking advice with experienced mentors, fostering collaboration regardless of location. Additionally, the continued success of the online DPM Training Programme and the Physician and Scientist Induction Programme, now in its third year, underscores our commitment to education and professional development.
In a sign that our members’ expertise is valued outside of our specialty, FPM was awarded a grant to develop an eLearning programme on respiratory diseases. The resultant Managing Respiratory Diseases programme was successfully launched in September 2024 and is an important resource for healthcare workers in the NHS.
Raising FPM’s profile and developing new relationships was also a key focus this year. Notably, we were invited to speak at the Middle East Association of Pharmaceutical Professionals’ second conference as well as the Pharmasteer Conference in India and we hosted a medical device clinician CPD day, highlighting the increasing convergence of medical devices and medicines.
Recognition from industry peers has further validated our progress, with FPM being shortlisted for multiple Memcom Excellence Awards and Association Excellence Awards, securing a win in both. These achievements are a testament to the dedication of our staff and volunteers in advancing the field of pharmaceutical medicine.
Education and Standards
2024 was a very productive year for Education and Standards, with some of the resourcing and operational changes of 2023 bedding down and some exciting initiatives launched.
Specialty training
Reconstruction was the theme of the year for specialty training. January saw the arrival of a new Specialty Training Manager and by early summer a backlog of queries and applications to join the specialty training programme or register new training sites had been addressed. 25 new doctors joined the programme in 2024, and the current overall number of trainees is 79, which is very encouraging.
Meanwhile, significant progress was made in restoring some of the governance structures around specialty training which had been facing challenges. February saw the arrival of a new Chair for the Specialist Advisory Committee (SAC), while the autumn saw work on reviving the dormant Trainees Committee come to fruition.
We also looked at how we could improve communications with trainees, and July saw publication of the first edition of PMST Focus, a new quarterly newsletter for current trainees, Specialty Advisors and ARCP panellists, among others. The newsletter included the first information on the updated PharmaTrain syllabus which will be rolled out in 2025–26. A second quarterly edition was published in October.
Training
The DPM Training Programme had an exceptional year, with 91 delegates on the programme (a growth of 30%) and income considerably ahead of forecast. The programme is designed to prepare candidates for the Diploma in Pharmaceutical Medicine (DPM) examinations and its success bodes well for our ability to bring people into FPM via training, through the exams and then into membership.
The programme also received industry recognition with the awarding of a prestigious Association Excellence Award in the Best Learning Programme category. It is notable that not all participants pursue the programme to prepare them for the examinations, with many completing the modules purely to enhance their professional development using the high-quality learning package.
Our small portfolio of one-day training courses attracted healthy numbers. Our ever-popular ABPI Code in a Day course ran three times and was joined by Pharmaceutical Medicine in a Day, a ‘taster’ course for those interested in what pharmaceutical medicine has to offer, and Understanding and Interpreting Real World Evidence, which is run in association with the Drug Safety Research Unit (DSRU).
Educating others
In September 2024, FPM launched the highly anticipated Managing Respiratory Diseases e-learning series. The series is the result of months of collaboration between FPM staff, subject matter experts, and an instructional design team and emerged from FPM’s Defining Medical Needs and Evidence (DEMENDE) working group. Designed for all healthcare professionals in the UK—including general practitioners, specialist doctors (particularly those in intensive care medicine, geriatric medicine, and oncology), and nurses—it is available free of charge on the NHS Learning Hub. The project was made possible through a grant from Pfizer Ltd; however, Pfizer Ltd had no involvement in the development of its content.
The undergraduate Drug Discovery and Development programme, developed in collaboration with the ABPI and Brighton and Sussex Medical School, and also delivered to Kent and Medway Medical School, entered its fifth year in 2024. This programme addresses a critical teaching gap, as many UK medical schools do not include comprehensive education on how medicines are developed and used in their undergraduate or postgraduate training for clinical doctors and other allied healthcare professionals (HCPs) in the NHS. By bridging this gap, the programme equips undergraduates with essential knowledge on the use of medicines across the NHS. Additionally, educating doctors and HCPs on the development, regulation, and accessibility of medicines fosters greater understanding and trust. The programme’s success has led to plans for a digital version, expanding its reach to more medical schools.
The Physician and Scientist Induction Programme was another success story this year, with the fifth and sixth sessions delivered for a US-based client. We also ran a separate course on drug development with the same client.
Examinations
2024 was a successful year for FPM’s examinations, not least with the arrival in March of a new Chair for the Office of the Board of Examiners (OBoE), the first since the departure of the previous Chair in late 2022.
As of the end of 2024 there were some vacant roles on OBoE still to be filled, but the arrival of a new Chair and new committee members following 2023’s recruitment drive helped to stabilise the work in this area. Once again, we are grateful to OBoE for their hard work and dedication to maintaining high standards during this period of transition.
The year also saw numbers for FPM’s examinations rise, with 89 applications being received, a higher number than in recent years, and they included applications from non-physicians who were for the first time eligible to sit the Multiple Choice Question paper for the Certificate in Pharmaceutical Medicine. Sixty-five candidates in total sat the CPM exam, of whom nine were non-physicians – a promising start. The number of candidates sitting the Diploma exams was also robust, at 38 for the Short Answer Questions paper and 28 for the Critical Appraisal Paper respectively.
In November we were able to reveal that the UK General Medical Council (GMC) had approved the DPM as an acceptable postgraduate qualification for International Medical Graduates who are seeking GMC registration – a significant milestone that acknowledges the quality of our flagship examination.
Policy and Communications
Stakeholder engagement
The policy and communications function of FPM primarily exists to ensure our members are well supported, and are informed and engaged with FPM activities. It also interacts with external stakeholders, including: government; third sector bodies; the life sciences industry and regulators; colleagues in academia and clinical healthcare; the press; and patients and the general public. In 2024 we continued to raise awareness of and advocate publicly for the science and practice of pharmaceutical medicine and the benefits that the speciality can bring to public health.
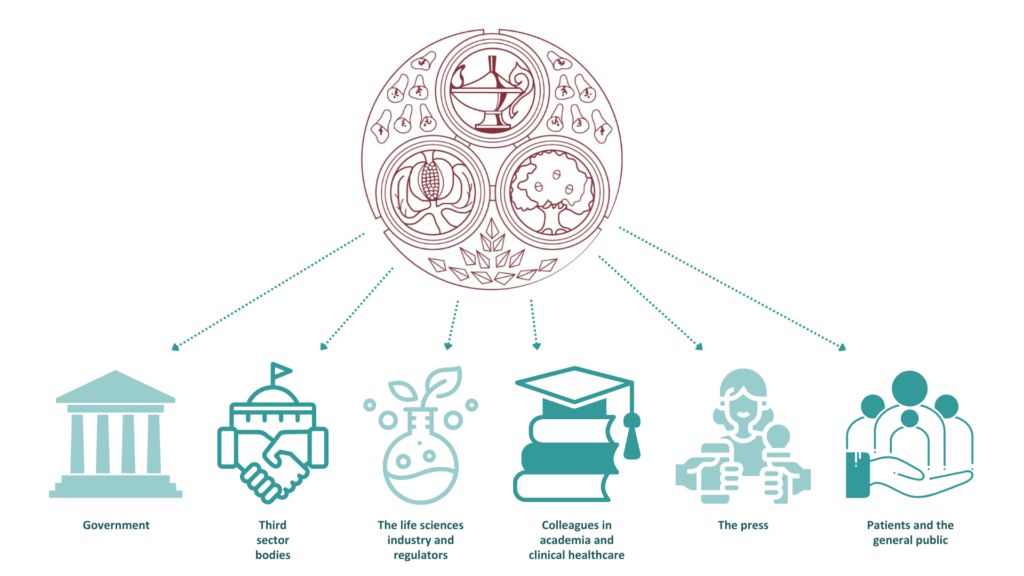
The FPM Policy and Communications Group, seven expert groups, EDI Forum, and Patient Partnerships Forum are comprised of members with a special interest and expertise in external engagement and extensive subject matter knowledge. The expert groups cover almost every aspect of pharmaceutical medicine, from oncology, through medical devices and other new technologies, to the regulations around the development and use of treatments. All these groups work alongside a professional staff team with expertise in digital content and strategy, editorial processes and public policy development.
Public policy in 2024
In 2024 our policy-oriented volunteer groups were particularly busy developing responses to several major public consultations, including: NHS England’s 10-year Health Plan, the PMCPA’s proposed changes to the ABPI Code of Practice, and the MHRA’s consultation on medical devices regulation.
Volunteers and staff worked on many thought leadership blogs throughout the year, including some which were timed to coincide with wider societal observances. These included a blog on data integrity in rare diseases published for February’s Rare Disease Day, an article on the role of AI in diagnosis published during Jeans for Genes week, and a World AIDS Day blog that reflected on 40 years of innovation.
 FPM also continues to play a very active role in collaborative engagement across the healthcare and life sciences ecosystem. In October we hosted a roundtable on environmental sustainability in the pharmaceutical industry that attracted participants from various disciplines and geographies. In November our newly-appointed President Dr Sheuli Porkess chaired a roundtable on diversity, a collaboration between FPM, Egality, and Paddington Life Sciences that brought together leaders in healthcare to discuss embedding diversity in all its definitions into clinical trial design. This event was a follow-up to a roundtable that FPM hosted in 2023 and there will be a report published based on the discussions.
FPM also continues to play a very active role in collaborative engagement across the healthcare and life sciences ecosystem. In October we hosted a roundtable on environmental sustainability in the pharmaceutical industry that attracted participants from various disciplines and geographies. In November our newly-appointed President Dr Sheuli Porkess chaired a roundtable on diversity, a collaboration between FPM, Egality, and Paddington Life Sciences that brought together leaders in healthcare to discuss embedding diversity in all its definitions into clinical trial design. This event was a follow-up to a roundtable that FPM hosted in 2023 and there will be a report published based on the discussions.
Raising FPM’s global profile
In 2024, FPM enhanced its global presence by fostering partnerships that promote best practices and cross-border collaboration, positioning itself as an international hub for physicians in the field. In the summer, our comms team worked with two US-based members to create short videos highlighting their experiences as pharmaceutical physicians in the US. These helped to dispel the notion that FPM is UK-centric. In September 2024 the Middle East Association for Pharmaceutical Professionals (MEAPP) invited Dr Flic Gabbay and Dr Sheuli Porkess (then President and Vice-President of FPM respectively), and Chief Executive Dr Marcia Philbin to address MEAPP’s second annual conference. In December our President, Dr Sheuli Porkess, presented at an India-based conference on Global Medical Affairs, and championed FPM’s position as a global authority.
Innovation in our digital communications
In 2024 our communications team drove innovations in brand design, and strategic communications across the organisation. Their work underpinned much of our outward-facing activities and included a multi-channel campaign to support the revised Fellowship eligibility criteria, the creation of a sub-brand for the FPM Annual Symposium, and a thorough review and refresh of our critically important PMST web pages.
They also redesigned our monthly email bulletin to improve clarity and engagement. The new format features a structured hierarchy, with a headline item followed by key notices, making it easier to navigate. The reach of the bulletin was expanded by repurposing its content as a LinkedIn Newsletter, neatly leveraging our most active social platform to engage a wider audience and maximise impact. Email campaigns remain the key driver of sales for FPM and it is gratifying that the FPM staff’s efforts in developing our email communications has yielded reward in income gains across all our key products and services.
The communications team has also been evaluating opportunities to reach new audiences via emerging communications channels, and has soft-launched profiles on Threads and BlueSky.
Open rates (%age) of email campaigns (source, Mailchimp (2024))
Equality, Diversion and Inclusion
Throughout 2024, FPM focused on enhancing diversity and inclusion within its membership, leadership, and the wider pharmaceutical sector, aiming to drive innovation and improve patient outcomes.
The FPM Equality, Diversity, and Inclusion (EDI) Forum continued engaging underrepresented groups. Blogs highlighted the link between diversity and innovation, with Dr John Bolodeoku and Dr Bu Siakpere stressing inclusive medical research and the role of pharmaceutical physicians in humanitarian crises.
We were excited to play a leading role in a cross-industry roundtable that discussed diversity in clinical trials, the report of which is due to be published in April 2025.
Finally, following a review of 2024 Fellowship applications, we sought to address gender imbalances by launching a gender bias survey to evaluate how we could encourage more women to apply for Fellowship. The insights from this survey were fed back into the 2025 Fellowship planning process.
Digital metrics from 2024
Events
FPM Annual Symposium 2024
Our flagship event was a major focus throughout 2024, culminating in a highly successful gathering in November. For 2024 we introduced significant enhancements to maximise its impact and accessibility. A refreshed brand identity and a revised booking structure—extending the reservation window—helped boost attendance and engagement. Additionally, we moved to a prestigious new venue, the Royal Society, providing an upgraded experience for in-person participants.
Our communications team worked to generate advocate marketing, successfully amplifying awareness of the event. This effort led to our esteemed keynote speakers, Dame Sally Davies and Professor Jonathan Van-Tam Kt, generously recording video messages ahead of the event to build anticipation.
The event itself brought together a diverse global audience of healthcare professionals and leaders from the life sciences community. Discussions spanned a broad spectrum of critical topics, including antimicrobial resistance, artificial intelligence, precision medicine, and patient engagement. Our distinguished line-up of speakers featured Professor Rory Collins Kt, Professor Chloe Orkin, Dr Amit Aggarwal, and many others, alongside Dame Sally and Professor Van-Tam.
To extend the event’s lasting impact, we produced a multi-author review blog and collaborated with a video production team to create high-quality content for the promotion of future events.
FPM Annual Symposium Highlights
Some of the feedback from delegates who attended FPM Annual Symposium 2024
This is the first time I have joined the online version of this meeting. It was excellent.
This was the best annual symposium I have ever attended, hats off to the organisers.
It was a great experience overall for me.
FPM Annual Awards 2024
The FPM Annual Awards took place in July 2024 at the RCP London and gave us an opportunity to celebrate achievements of members and other colleagues in the life sciences who have made significant contributions to the field of pharmaceutical medicine.
The FPM President’s medal, which is the highest honour FPM bestows, was awarded to Dr David Jeffreys in recognition of his many years’ significant contribution to the specialty of pharmaceutical medicine. Dr Renate Crome, meanwhile, was the recipient of our Volunteer Award which is given in recognition of outstanding contributions that have helped FPM achieve excellence in advancing the science and practice of pharmaceutical medicine. The academic achievement awards, given to the candidates who scored the highest marks in the DPM Part 1 and DPM Part 2 examinations respectively, were both awarded to Dr Rory Taylor. Honorary Fellowships were bestowed on Dr Carl Naraynassamy, Professor Maggie Rae and Dr John Rex, and Honorary Membership was awarded to Ms Magda Chlebus. Finally, 62 new Fellows were admitted, the first cohort under our revised criteria, and we were delighted to see such great interest.
Fireside chats and other events
Our two flagship events were supported by additional smaller events which gave members and others regular touchpoints with FPM. These included a fireside chats to mark World Mental Health Day and International Women’s Day, a Journal Club event, an Examiner Training Day, and an ABPI Code Consultation response discussion.
Celebrations for FPM
In late 2024 FPM received recognition at two industry awards ceremonies. In September we were thrilled when our Deputy Chief Executive Will Booth won the coveted Memcom Future Leader Award 2024. This prestigious award recognises emerging leaders in the membership sector who demonstrate exceptional leadership potential. After his win, Will commented “I’m stunned… just stunned. Thank you so much to the judges this year and to all my colleagues – it’s an absolute pleasure working with you!”
In November we are delighted when our DPM Training Programme received a silver award for Best Learning/Professional Development Programme (up to 2000 members) at the Association Excellence Awards 2024. Judges cited it as an example of successful integration to bring training ‘‘in-house’ through a mix of external funding and own investment”.
Finally, in the summer, our Chief Executive Dr Marcia Philbin was recognised in Management Today’s Women in Leadership Power List 2024, acknowledging her exceptional vision and leadership.
The FPM Designated Body
FPM is a Designated Body for providing annual appraisals and GMC revalidation. As of 31 December 2024, 707 members had a prescribed connection to FPM as a designated body, which is 44% of FPM members. This is highest number of connected doctors since the introduction of revalidation at the end of 2012. In 2024, 91 doctors connected to the DB, which is 13% of the total number of connected doctors.
Following a successful recruitment drive for new appraisers, we held two New Appraiser Training (NAT) sessions and trained 17 new appraisers. This means that we are fortunate to now have a total of 97 highly enthusiastic appraisers. Feedback from the NATs was extremely positive, and we are delighted that our appraiser pool has expanded to meet the rising demands of our growing number of connected doctors. Increasing appraiser numbers has allowed for greater flexibility of allocations and a greater capacity for urgent appraisals. We always ensure we have appraisers who are connected to other designated bodies including, for example, the NHS. This helps to ensure similar standards across pharma and benchmark with other designed bodies. We have continued to hold quarterly meetings with the three Appraisal Leads which are valuable for ensuring consistency and for generating quality improvement ideas. The Appraisal Leads also deliver an online introduction to appraisal and revalidation every six weeks mainly for newly connecting doctors but it is open to all connected doctors. This year, we provided four networking sessions for our appraiser team, which took place both in-person and online. Attendance at these non-mandatory sessions was so popular that we added a fifth session to meet the demand. In total, 74 appraisers attended, and the feedback received was very positive.
The most recent ratings and free text comments from the post-appraisal questionnaire which appraisees complete are once again overwhelmingly positive. This includes views on the appraisal platform PReP and personal feedback on the appraiser. The results are aggregated for each appraiser and sent to them annually. Importantly they show that doctors felt well supported by their appraisers and the office team. It was clear that many felt that having the opportunity to discuss the events of the year with a peer was invaluable.
Future Plans
In 2025, the focus will be on developing the new strategy for 2026-2028. As we develop the new strategy, work will be undertaken to look at the future of pharmaceutical medicine and the needs of its members. This will inform how FPM can position itself to serve the public.
Furthermore, in line with good practice, a governance review will be undertaken to modernise how FPM is governed, decisions are made as well as provide clarity on roles and responsibilities guided by the FPM values.
The lease of FPM’s current office ends in November so work is underway to find, agree and relocate to new offices in London. Since the COVID-19 pandemic, the way we work has changed with staff working flexibly and member visits limited to attending specific meetings. This is an opportunity to move to an affordable space which offers the flexibility that meets our operational requirements as well as balances the need for teams to work together. This will be a significant undertaking and a detailed project plan has been drafted to help minimise the disruption to day to day operations during the move.
Unfortunately, the planned review and revision of the DPM examination question and standard setting processes was not undertaken in 2024 due to workload so this will now be carried out in 2025 with a view to deliver at least two diets of examinations per annum from 2026.
Thank You
Finally, once again FPM would like to extend thanks to all our members who contributed to our activities in 2024, whether as committee members, examiners, specialty advisers, educational supervisors, appraisers or by supporting raising awareness and advocacy events and policy projects. We truly value your participation and support.
Remembering Hamilton
 Very sadly our office dog and ‘Chief Morale Officer’, Hamilton, was put to sleep on 12 September after a period of ill health. He had been a regular face in the office and on zoom calls for six years and is missed by everybody at FPM.
Very sadly our office dog and ‘Chief Morale Officer’, Hamilton, was put to sleep on 12 September after a period of ill health. He had been a regular face in the office and on zoom calls for six years and is missed by everybody at FPM.
Hamilton belonged to our Head of Marketing and Communications Will Strange who thanks colleagues and FPM guests for the kindness they always showed him. Hamilton’s twin loves of making friends and raiding bins made his office days some of his favourite times.









