FPM Annual Report and Accounts
For the year ending 31 December 2021
Forewords
From the President and the Chief Executive

COVID-19 dominated all pharmaceutical physicians’ activities in 2021, whether it be specifically in running clinical trials to bring more COVID vaccines and therapeutics to patients, trying to maintain the pipeline of medicines in development for other conditions, or ensuring the safety and accessibility of medicines already approved for use. The work of the Faculty of Pharmaceutical Medicine (FPM) has also inevitably been touched by the pandemic in every way throughout the past year. However, mirroring the endeavour, tenacity and flexibility of our individual members, FPM has continued to adapt and innovate in the delivery of all our activities and has tackled the pandemic head-on with policy and advocacy work.
As 2021 dawned, COVID vaccines were rolled out, the new variants were less severe and lockdowns became less extreme, but returning to “normal” life has been a challenge for all, including our own office, with constant opening and closing, and rules, regulations and social attitudes making it very challenging to plan and schedule events and activities. Despite this, we have had many achievements across the departments of FPM, as you will see from this report. Whilst the pandemic has undoubtedly raised our visibility, it has also brought into sharp focus that we are a small organisation with only 1500 members. This means that representing pharmaceutical medicine to policymakers at national level can be extremely challenging, but is nevertheless critical to delivering vaccines, medicines and devices that ensure all patients get the maximum chance of a healthy life.
High standards in the education, training and continuing professional development of pharmaceutical physicians is essential and a core aim of FPM. 2021 saw the culmination of a long-term project to revise the Pharmaceutical Medicine Specialty Training (PMST) curriculum, which received GMC approval in May. This is a tremendous milestone and a tribute to all those involved. FPM’s examinations have remained robust and we have also introduced the new Diploma in Pharmaceutical Medicine (DPM) training programme, which is delivered online to candidates around the world. In 2021 we admitted 35 new Members (MFPM), most of whom have taken and passed the DPM and many are also enrolled in PMST. A special mention must go to the winner of the 2021 FPM ‘President’s Medal’ – Dr Susan Bews – to whom we owe so much for establishing our specialty.
In November 2021 I took over as FPM President and was immediately dropped into regular meetings with ministers and senior staff from the Department of Health and Social Care and NHS England, as we faced yet another COVID wave. These meetings have been open and two-way exchanges and I am hugely grateful to the support I have received from members of FPM, especially those in companies with vaccines and treatments for COVID-19 and in regulatory agencies. 2020 and 2021 have marked a turning point for both the specialty of Pharmaceutical Medicine and FPM itself. We, along with clinical colleagues, have been challenged, but have shown just how much we can achieve through innovation, collaboration and sheer hard work. There are certainly more challenges to come in the year(s) ahead, but I look forward to an exciting time working with all FPM members, staff and stakeholders to achieving on our mission: To advance the science and practice of pharmaceutical medicine for the benefit of the public.
FPM’s highlights in 2021
Board of Trustees' Report
The trustees are pleased to present their annual report together with the audited financial statements for the financial year ended 31 December 2021. The financial statements comply with current statutory requirements, the Memorandum and Articles of Association and the Statement of Recommended Practice – Accounting and Reporting by Charities (SORP FRS 102).
Our purpose
To advance the science and practice of pharmaceutical medicine by working to develop and maintain competence, ethics and integrity and the highest professional standards in the specialty for the benefit of the public.
Public benefit
The charitable purposes of Faculty of Pharmaceutical Medicine (FPM) are set out in the Memorandum and Articles of Association and are:
- to promote the science of pharmaceutical medicine.
- to develop and maintain competence, ethical integrity and high professional standards in the practiceof pharmaceutical medicine; and
- to advance knowledge in pharmaceutical medicine.
Pharmaceutical medicine is the medical specialty concerned with the discovery, development, evaluation, licensing and monitoring of medicines and the medical aspects of their marketing.
FPM seeks through its activities to bring about an improvement in the health of the public and patients. Our activities seek to advance the science and practice of pharmaceutical medicine by contributing to the provision of effective medicines for public benefit. The trustees regularly review the aims, objectives and activities of the charity referring to the Charity Commission’s guidance on public benefit.
Our vision
A world where effective medicines meet the needs of patients.
Our mission
To advance the science and practice of pharmaceutical medicine for the benefit of the public.
We will do this through four strategic priorities:
- Set and appraise standards for training in and practice of pharmaceutical medicine
- Promote understanding of pharmaceutical medicine to create trust
- Engage with clinical doctors to promote pharmaceutical medicine as a career option and support all FPM members in their training and practice
- Ensure good governance and financial stability
The delivery of the strategic objectives will be guided by the following values, which will guide staff and members’ behaviour:
| WE ARE: | THIS MEANS: |
|---|---|
| Professional | Being accountable for our work and actions |
| Innovative | Seeking solutions proactively |
| Caring | Treating everyone with dignity |
| Collaborative | Working positively with others |
| Credible | Being honest and ethical in our work |
| Learned | Investing in developing knowledge and skills |
Movers and Shakers
2021 was a year of change for FPM. There was a new President, Vice President, Registrar and three new trustees.
In addition, new staff numbers were recruited to reflect the breadth of new work now being undertaken in FPM. Indeed, it has been a busy and productive year for FPM so let’s take a trip down memory lane to see what else we achieved in 2021.
Policy, Communications and Ethical Best Practice
The FPM policy and communications team continues to raise awareness of the science and practice of pharmaceutical medicine, advocate the power of the specialty and the benefits it can bring to patients, and promote and support a greater understanding of and trust in medicines.
Supporting understanding in COVID-19
During 2021, FPM has played a significant role in educating and informing the public, mainly regarding the launch of COVID vaccinations and treatments.
We have also engaged with our clinical colleagues in the NHS with regard to optimising prescribing and the utility of treatments. As new COVID variants have emerged, FPM published detailed analyses, which have been used as fundamental resources for policymakers and journalists.
One of the most critical concerns of COVID-19 was keeping clinical trials going and ensuring patients’ data from trials was utilised effectively. Early in 2021, we undertook a survey and convened a workshop of FPM members, which resulted in the Clinical Trials Resilience report, which made recommendations for future adaptations, innovations and best practice. As the year came to a close, and as the Omicron wave hit and the new antivirals emerged, FPM was invited to directly support decision makers in the Department of Health and Social Care and central Government. This has given impetus to FPM leading a policy and research project examining the future of antiviral research and innovation, and how we manage pandemics and deploy treatments to patients, especially ensuring equality of access.
Driving the women’s health agenda
As part of FPM’s mission to advance the science of pharmaceutical medicine for the benefit of all patients, we continue to highlight areas of unmet medical need and communities who are sometimes overlooked and marginalised in healthcare decision making.
A big focus of this throughout 2021 has been in the area of women’s health – an often neglected area of medicines development.
The chair of the FPM Policy and Communications Group – Dr Allyah Abbas-Hanif – has been a commissioner on Safe, Effective and Accessible Medicines in Pregnancy: A Call to Action, to which several of our members gave evidence. In 2021 FPM conducted a survey of our membership for the DHSC
Women’s Health Strategy consultation and submitted a comprehensive response. We also held a series of free-to-attend open webinars on drug development for the menopause, which included discussion of what is on the horizon for new treatments. Outputs from this exercise have informed a subsequent submission to the development of the new NICE guidance on the menopause.
In April 2021 Flic Gabbay was asked to present at the Venice Forum: Maternal, Newborn and Child Health. This is a major international conference on public health issues in this field and the paper explored “what innovations and challenges do you see for pharmaceutical development and clinical trials in the MNCH space for the future?” The presentation was subsequently followed up with a brief editorial in the Lancet.
Collaborations and flying the flag for pharmaceutical medicine
FPM’s relationship with external bodies continued to grow and strengthen during 2021. Maintain our longstanding partnership with the British Pharmacological Society and ABPI on the Clinical Pharmacology Skills Alliance, we also grew our influence with the Academy of Medical Sciences’ (AMS) ‘FORUM’ and their programmes of events and activities.
FPM Vice-President – Dr Flic Gabbay – was appointed a Fellow of the AMS and subsequently invited to be part of two working parties on antimicrobial resistance and new antibiotics. FPM partnered with the AMS FORUM to organise a workshop on clinical trials in rare diseases.
In December, Dr Gabbay was invited to be part of a panel discussion organised by the National Academies: the Academy of Medical Sciences, the British Academy, the Royal Academy of Engineering, and the Royal Society. The panel was chaired by Sir Patrick Vallance and discussed the UK Prime Minister’s ambitions in ‘Delivering Strategic Advantage in Science and Technology’. Dr Gabbay spoke about the public health elements of medicines development and science.
Not forgetting medical devices and diagnostics
In the same way that vaccines, medicines and clinical trials became part of the everyday lexicon during COVID-19 pandemic, medical devices and diagnostics too, in the shape of LFTs, PCRs, ventilators etc, have also been discussed in public like never before.
This public discussion is mirroring an incredible surge of innovation and growth across devices, diagnostics and the use of artificial intelligence to support diagnosis and medicines development. These technologies can allow patients to get access to appropriate medicines, preventing adverse events and as digital devices controlling effective delivery.
Many FPM members work in these fields and the FPM’s Medical Devices, Diagnostics and New Technologies Expert group, led by Dr Bob Holland, has been working to embed education, information and understanding amongst our community, both internally and externally. Most notably, the group led the development of the FPM’s response to the consultation on MHRA Future of Medical Device Regulation.
FPM’s publishing output
FPM’s publishing output continued to thrive in 2021 with a plethora of ‘Deep Dive’ articles and blogs, covering everything from nitrosamines in pharmaceuticals and therapeutics for COVID-19, to the globalisation of medicine and African medicine, patient involvement and the impact of COVID-19 on women’s health.
These are supported by regular Bulletins, which keep members informed and up to date, and retain very high levels of engagement versus industry standards.
| FPM E-bulletin | Professional Services | Pharmaceuticals | Non-profit |
|---|---|---|---|
| 45% Open Rate | 22% Open Rate | 19% Open Rate | 25% Open Rate |
| 9% Click Rate | 2.5% Click Rate | 2.3% Click Rate | 2.8% Click Rate |
FPM continues to engage an expanding audience via social media, delivering thought leadership, links to articles, and posts of FPM statements, event notices and other activities. The FPM LinkedIn page remains a key tool in our digital communications. This audience has been developed organically and the most popular posts have been ones that have celebrated achievements of our members e.g. during the Annual Awards in January. Our Twitter presence is also being amplified by increasing support from key stakeholders.
Ethics, best practice and sustainability – delivering the best for public health, patients and the planet
In terms of professional practice, the Ethics and Practice Committee (EPC) supported a series of three webinars designed to help medical directors and other leaders in embedding the ABPI Principles within their organisations.
The sessions focused on: an Introduction to the ABPI Principles; the ‘Patients’ principle; and the Integrity, Transparency and Respect principles. We are now considering how best to bring FPM’s Good Pharmaceutical Medical Practice guidance ‘to life’ and make it as useful and engaging as possible for FPM members, and the structures and processes required to achieve this.
We have also begun discussions on how to develop the EPC to encompass the principles of ESG (environmental, social, governance), to ensure that the work of FPM is fit for purpose, and to support our members to embed these principles in their everyday practice. Demonstrating our commitment to environmental sustainability, FPM was invited to participate in a panel discussion on the Decarbonisation of Medicine which was organised by NHE365 and hosted by the TV presenter and journalist Helen Fospero.
Other highlights
Two other highlights of the year must be FPM’s success at the 2021 Memcom Awards and our Vice-President’s delivery of the RCP children’s Christmas Lecture.
At the Memcom awards FPM was shortlisted across the PR and communications categories and won the ‘Highly Commended’ award for the 2020 FPM Annual Symposium. This is recognition of a combined effort across the department, with staff and FPM members embodying the true spirit and values of a successful membership organisation.
The Royal College of Physicians’ (RCP) children’s Christmas lecture was delivered on 22 December by Dr Sheuli Porkess, FPM Vice President. In the talk, entitled Drugs and Bugs, Sheuli explained how medicines can be used to fight diseases caused by bugs, why antibiotic resistance is an issue and what young people can do to help. The webinar, aimed at 12-18 year-olds, attracted 200+ viewers from around the world.
Building the team
In order to successfully adapt to evolving challenges and expectations, the policy and communications team has grown and brought in two new staff members.
A Policy and Press Coordinator was recruited to support policy administration as well as press and media engagement, and a Digital Communications Assistant, as part of the new FPM Develop internship scheme, was on-boarded to support the ever-growing communications and social media activities.
Events
The COVID-19 crisis has accelerated FPM’s use of digital platforms to broaden the range of events that it offers its members, which include offering both free and paid-for webinars for educational purposes, training programmes and special events.
We have collaborated with three organisations in 2021 to develop and run specific programmes:
- Drug Safety Research Unit (DSRU) – Understanding Observational Studies in the time of COVID-19
- Medical Women’s Federation (MWF) – The Menopause: Not just a woman’s issue
- Association of the British Pharmaceutical Industry (ABPI) – Code of Practice Principles.
The success of the online events means that FPM will in future develop a model of both face to face and online activities with the aim of increasing accessibility to all its members, as well as non-members.
Another development in the events ecosphere was the launch of FPM On Demand, which allows members and non-members to catch up on past events at a time convenient for them. As many of the events are eligible for CPD, this provides pharmaceutical professionals with opportunities to develop their knowledge and extend their skills.
| Education | Training | Conversations | Specific Topics |
|---|---|---|---|
| FPM Annual Symposium 2021 | Managing Medical Emergencies | Enabling a Greater Diversity of People in Clinical Trials | DSRU - Understanding Observational Studies in the time of COVID-19 |
| FPM Education Week 2021 | The Code in a Day | In Vitro Diagnostics – Companions or Not? | ABPI-FPM Joint Events | Integrity, Transparency and Respect: the ABPI Code of Practice Principles |
| Preparing for the DPM Exam Q&A | Essential Leadership Skills for Pharmaceutical Physicians | Fireside chat with Pauline Williams CBE | |
| DPM Training Programme | Professional wellbeing workshops | FPM x MWF – The Menopause: Not just a woman’s issue | |
| Fireside chat with Bu Siakpere and Sheuli Porkess |
FPM Annual Symposium
‘Trials and Tribulations – Shaping a Bright New Future for Pharmaceutical Medicine’
Our November Symposium is our flagship event and an important opportunity for our members to feel connected to their professional body and to each other.
This year we continued to welcome high profile speakers including: Dr Özlem Türeci, Dr Stephen Lockhart, Prof Jonathan Van-Tam, Dr June Raine, Prof Helen McShane, Cristina Durán and many others.
During the event several key themes came to the fore: communication and its importance in forging relationships between biotechs, big pharma and public health: the power of collaboration and a call to build on the collaborative successes of the pandemic: and the game changing power of AI and machine learning.
The virtual medium once again allowed us to welcome speakers and attendees from all over the world, as well as host people who are not able to attend in-person events, whether during a global pandemic or in ‘normal’ times. Going forward, we will run the Annual Symposium as a hybrid event so that we can continue to provide access to all.

“[Trials and Tribulations] brought home the huge potential for Pharmaceutical Medicine to collaborate,
innovate and shape a bright new future across clinical trials, regulatory, access to medicines and much
more.”

Education and Standards
Education and Training
It has been a busy year for education at FPM, with several appointments to the team, including Samantha Baglioni as Head of Education in March, Kay Grimwood as Exams and Standards Manager (maternity cover) in December, and Eve Snare as Education Manager in December.
Professor Penelope Ward was re-elected for another three-year term as Chair of the Education and Standards Committee in October. In September, other FPM Educational posts were taken up by Dr Ulrike Lorch as Director of Human Pharmacology and Dr Don Nwose as Director of Experimental Therapeutics.
DPM Training ran online successfully for a second year, attracting a high number of bookings across the nine modules. Professor Alan Boyd stepped down from leading the programme at the end of 2021 and a new Training and Development Director position was advertised in December, with Dr Sharon McCullough accepting the post early in 2022.
As part of her role, she will oversee the future development and delivery of DPM Training and contribute to the development of other educational programmes including the master classes and skills-based courses that are being planned for 2022.
The lecture-based undergraduate Pharmaceutical Medicine Roadshow for Brighton and Sussex Medical School (BSMS) and Kent and Medway Medical School (KMMS) continued to be delivered as live sessions, via remote communications applications. Other medical schools have expressed interest in the series and the partners – FPM, ABPI and BSMS – are considering how to convert the existing programme into a digital format to distribute the learning more widely. Grant funding to support the digitisation project will be sought in early 2022.
The effort to digitise and deliver FPM’s educational offering through a new learning management system (LMS) was progressed in November 2021 with the issue of a Request for Proposal (RFP) to a number of suppliers. Three vendors submitted by the deadline of the end of 2021 and the selection and implementation of a new system should take place by Q3 of 2022.

Pharmaceutical Medicine Specialty Training (PMST)
An important milestone and success for FPM was the GMC approval of FPM’s new ‘Curriculum for Pharmaceutical Medicine Speciality Training’ on 20 May, which was formally implemented in August.
Of the 100 trainees enrolled on the programme, 39 were transferred to the new curriculum and any new entrants will embark on the new curriculum. As part of a PMST marketing plan, several ‘PMST Champions’ have been trained to promote the specialty as a route to becoming a licensed pharmaceutical physician, demonstrating high professional standards with integrity and trustworthiness.
In 2021 23 trainees completed the programme, there were 16 new enrolments in the same period and 23 new CCTs were awarded by the GMC.
The GMC held its annual national training survey in May. A total of 52 pharmaceutical medicine trainees (65%) and 51 educational supervisors (64%) completed the survey. The overall satisfaction of trainees with their training programme was 81%, down 4% from 2019; the overall satisfaction of educational supervisors in their role was 72%, which was unchanged from 2019.
FPM is piloting a new two-stage enrolment process, which now features an assessment day where applicants are assessed on presentation delivery and interview performance. The first of these was held in September for which we received nine applications, six of which were held, with three applications deferred to the second assessment day in December due to a limit on available spaces.
In October, our first four-year quality assurance cycle with the GMC began with the submission of an annual self-declaration by Professor Geeta Menon, the postgraduate dean and responsible officer for the Pharmaceutical Medicine Deanery.
PMST is your route to professionalism in Pharmaceutical Medicine
Examinations
The Certificate in Pharmaceutical Medicine (CPM) / Diploma in Pharmaceutical Medicine (DPM) and Diploma in Human Pharmacology (DHP) / Certificate in Human Pharmacology (CHP) exams were held remotely again in 2021 using the TestReach system and remote invigilation.
The CPM and DPM exams were held in September and October, respectively, and the CHP and DHP exams were held in October.
As FPM’s exams are now online the number of international exam candidates continues to grow with candidates from India, Australia, Japan, Turkey and Germany in 2021.
Issues with the exam system were minimal this year and benefited from a series of improvements introduced by the supplier, who collaborated with FPM to understand how to create and edit questions in-house, within the system.
Volunteers from the examiner group participated in question setting panels, organised with the aim of building up the bank of questions in particular areas: healthcare marketplace; pharmaceutical development; early development, and to add any new topics. The Office of Board of Examiners (OBOE) intends to continue the panels in early 2022.
DIPLOMA (DHP) and CERTIFICATE (CHP) IN HUMAN PHARMACHOLOGY
- 5 candidates sat the CHP exam; 4 candidates passed
- 4 candidates sat DHP exam (paper 2); 2 candidates passed
- 4 candidates sat the DHP exam (paper 3); 2 candidates passed
- 2 candidates were awarded the DHP qualification
Revalidation and appraisals
FPM is a Designated Body for providing annual appraisals and GMC revalidation.
On 31 December 2021, 629 members had a prescribed connection to FPM as a designated body. Due to COVID-19, appraisals were suspended for a six-month period during 2020 under the guidance of NHS England. Following the reintroduction of appraisals in October 2020, the team successfully ensured the full years’ worth of appraisals (579) went ahead within the six-month period up to March 2021. Feedback on the supportive nature of appraisals has been hugely positive. The staff group was strengthened with the appointment of a new Revalidation Coordinator in November 2021.
Membership Support
FPM is a professional membership organisation with members who are practising pharmaceutical physicians or those with a professional interest in the specialty.
FPM’s new Membership Committee was set up in 2021 to focus exclusively on our membership. Their remit includes growing our membership, extending and improving the benefits and services we provide, and reviewing the types of membership we offer along with their associated entitlements and privileges.
Membership projects include:
- Identifying ways to promote the specialty and careers in pharmaceutical medicine along with improving the careers information, guidance and support FPM provides
- Engaging with our membership to explore the value of membership, why they became a member and what benefits and services they value most
- Improving accessibility to Membership (MFPM) by extending the list of recognised qualifications accepted for MFPM as well as improving awareness of Membership by Distinction as a route to
MFPM
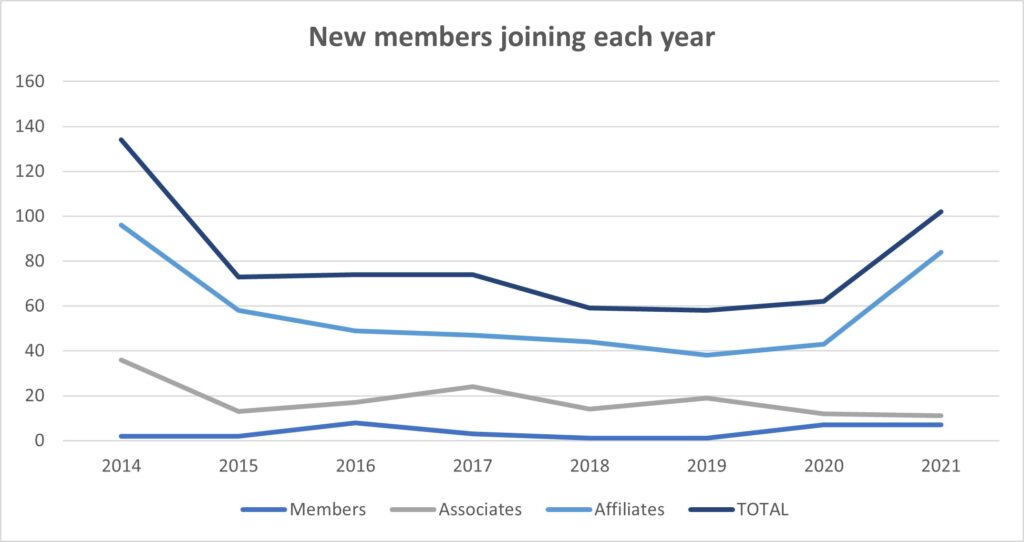
On the 31 December 2021 FPM had a total of 1,534 members in all categories and 1411 fee paying members, an increase of 3% on 2020. In 2021, 20% of our members were based outside the UK in over 36 countries, a similar percentage to 2020.
Despite the challenges over the last two years with COVID-19, for the first time since 2014, FPM saw a net growth in its membership numbers. FPM welcomed over 100 new members in 2021 especially from outside of the UK.
In 2021 we also saw an increase in members applying to upgrade to both Membership (MFPM) and Fellowship (FFPM) demonstrating that our membership is committed to FPM, a positive indicator for our long-term sustainability.

Awards and celebrations
In 2021, the FPM President’s medal was awarded to Dr Susan Bews.
Dr Bews has been pivotal in developing our structures and processes for appraisal and revalidation. Her service and commitment to the aims and mission of FPM, the establishment and development of our designated body, and the development of our specialty are immense and are duly recognised with the award of President’s medal.

For the first time, FPM won a Memcom Award.
Memcom is UK-based community of membership organisations, trade associations and those from the wider not-for-profit sector where senior leaders come together to collaborate, innovate, and learn from each other. FPM became a member in 2020. Each year Memcom holds the Excellence Awards and in 2021 for the first time, FPM submitted six entries and was shortlisted for three:
- Best Virtual or In person Membership Event of the Year – Best Educational Event (six shortlisted in total including the Royal Aeronautical Society)
- Best website (six shortlisted in total Including Royal College of Psychiatrists)
- Outstanding contribution to a membership organisation – Dr Penny Ward (four shortlisted in total)
To be shortlisted is itself a tremendous achievement for FPM as it signifies how our peers recognise the extent of our impact. FPM won the Highly Commended ‘Best Event of Year Award’ for its 2020 Annual Symposium – an important recognition of what staff, volunteers and members have achieved during this unprecedented era.

EDI Forum
The formation of the Equality, Diversity and Inclusion (EDI) Forum signified the importance that FPM places on supporting the breadth of its membership.
Considering the Forum first met in the early part of 2021, its output has been impressive, including three blogs, three Fireside chats and a video of a conversation on race and ethnicity which so far has garnered 1500 views on YouTube.
FPM was awarded £10,000 by the Royal Society of Chemistry to undertake a project called Women in Pharmaceutical Medicine to identify the barriers that may hinder the career progression of women. A special workstream was funded to explore the intersection of race and gender that affects black women in particular. The report is in the final stages of production and will be launched in 2022.
The work of the EDI Forum has not gone unnoticed by other professional bodies and the Chief Executive
was invited to speak at a meeting of the Intensive Care Society on 21 October as they were in the process
of forming their own EDI forum and wanted to learn from FPM.
Strengthening governance
The governance of FPM continues to be strengthened and modernised to reflect the changing environment in which we operate.
Three new committees were established to support the widening work of FPM.
- The Membership Committee was established to focus on the development of routes to membership, membership categories, the membership proposition and support for careers in pharmaceutical medicine across all career stages.
- FPM Global was established as the forum for members working outside the UK to address their specific needs as well as focus on local initiatives to promote the specialty of pharmaceutical medicine.
- The EDI Forum was established to address the diversity of FPM’s membership and advocate for equality, diversity and inclusion across the sector.
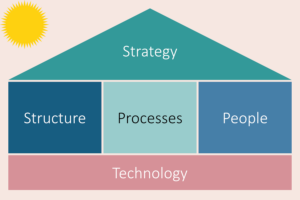
To further strengthen governance against the backdrop of the COVID-19 pandemic, FPM’s House of Agile was developed based on McKinsey’s “The Five Trademarks of Agile Organizations”, which outlines ways for organisations to transform from traditional ways of working to new ways and allows them to react quickly to changing circumstances caused by disruptions such as the pandemic 1,2.
FPM’s dynamic strategy is the roof of the house, the North Star that is steering the organisation through the intricate gauntlet of possibilities that has emerged as a result of the pandemic. The foundation of the house is the underpinning digital infrastructure and technology that enables FPM to maintain and modernise its business operations. The two pillars that strengthen the House of Agile are the team structures and the staff. Integrating these elements are the revised processes and operations that
sustains FPM’s operations.
Meet FPM’s staff group
The breadth of work undertaken in FPM is led by FPM staff who work collaboratively with members, stakeholders as well as with each other to ensure delivery against the strategic plan.
COVID-19 global pandemic - FPM’s response
The effects of the COVID-19 pandemic continued into 2021 and FPM, like many organisations, had to manage the logistical challenges of the ongoing disruption.
In spite of these significant tests, FPM has continued to thrive, and we have sustained and developed our routine activities during the year, as well as also tackling COVID head on, through public health information and advocacy work.
The growing scope of work has resulted in the expansion of the staff group as FPM continues to embrace new opportunities to widen its income streams, deliver more activities and collaborate more widely. FPM participated in the government’s Kickstart scheme to provide two unemployed young people with work experience, which has resulted in one of them being appointed to a permanent role.
We have developed our workplace environment to make it safer and more comfortable for staff and visitors and improved the terms and conditions of employment for staff. Hybrid working, trialled during the early stages of the pandemic, has now been introduced permanently. Air purifiers have been installed throughout the office and meeting rooms, and the office layout has been repurposed to accommodate the increasing staff numbers, whilst maintaining adequate space.
Our policy and communications work in direct response to the pandemic is described in detail above. We have continued to support FPM members in their work during the pandemic, and informed and guided policymakers and the public in their decision making through a plethora of media statements and comments, blogs and articles, and meetings and events.
The adoption of online working has helped FPM to increase engagement with its members. For example, the virtual Examiner training day on 5 March had the highest turnout with 17 potential new examiners and 10 examiners attending for refresher training.
FPM’s innovative events portfolio is another significant success story and an area where the changes forced by COVID have brought about adaptations, additions and refinements that have spectacularly improved our offering. Prior to 2020, FPM delivered 12 in person events each year, namely, The Annual Symposium, the Education Day, a half day workshop by the PCG, as well as leadership and professional training events. Now, FPM delivers 25-30 events covering thought leadership, engagement, training, education and professional development across all functions, both online and in person.
Future Plans
Unlike 2020 and 2021, the year 2022 will be dominated not only by the continued response to the COVID-19 pandemic, but by the tragic events unfolding in Ukraine, the energy crisis that will increase operating costs, and the impact of decisions arising from Brexit.
However, 2022 marks an important milestone for FPM, as it is the 20th anniversary of the launch of FPM’s first training programme in pharmaceutical medicine as a medical specialty. We plan to build on FPM’s existing track record of advocacy on behalf of pharmaceutical medicine and have created a new cohort of engaged members to be known as Champions, who will advocate clearly and consistently for pharmaceutical medicine specialty training (PMST).
As we come to the end of the current strategy, FPM is preparing the way forward for its sustainable growth and will focus in 2022 on developing its new 2023-2025 strategy. In addition, FPM has embarked on its digital modernisation project which will see the implementation of a new customer relationship management (CRM) system, a new web-portal to improve the interface between the CRM and website and a new learning management system (LMS) to support the ongoing development of the education
and training function. These developments will enable FPM to improve the efficiency in the delivery of its operations, as well as support the engagement and experience of its members’ interactions with the organisation.
In early 2021 FPM recruited to the new post, Head of Education, which was established to develop new education, training and assessment programmes that will strengthen FPM’s role in setting standards in pharmaceutical medicine. Through the development of multi-delivery educational channels that complement the traditional face-to-face delivery, FPM will now be able to offer a diverse range of educational programmes. The new Director of Training and Development, Dr Sharon McCullough, will lead on the development of the DPM Training programme which will include bespoke masterclasses.
The update of the curriculum for the specialty training programme was approved in May 2021 and work is ongoing to embed the new governance and operational frameworks. In addition, a marketing campaign has been developed to promote PMST and the benefits of undertaking the training, with a target to increase the number of trainees consistently over the next three years.
FPM is collaborating also with the Royal College of Physicians (London) to produce an e-learning programme that will introduce general clinicians and healthcare workers in the NHS to research methods and medicine development. This follows a successful bid into an NIHR competition valued at £120k per award. There has been an increase in awareness and appreciation of the importance of medicines development because of the pandemic and FPM will be able to showcase the expertise of its members
through this important collaboration.
In terms of policy and communications, we will continue to advocate for the development of safe and effective medicines, for an understanding of and trust in medicines, and to ensure that all patients have appropriate and equitable access to medicines and healthcare, and are able to be involved in clinical trials, should they wish. Our main areas of focus will continue to be development and deployment of vaccines and therapeutics for COVID-19, rare diseases, women’s health and leading the way in medical devices and diagnostics.
The membership project is another significant development for FPM and will focus on increasing FPM’s membership numbers, extending and improving the benefits and services we provide, and reviewing the types of membership we offer along with their associated entitlements and privileges. The success of the project will be evidenced by increased membership numbers across all categories, the depth of member engagement, such as those who volunteer to support the breadth of work in FPM, the support offered to all members at different stages of their careers and the financial sustainability of FPM.
The modernisation of FPM will see it continue to grow and increase its influence through the promotion of pharmaceutical medicine as an important medical specialty that has a pivotal role to play in combatting future health pandemics.
Thank you
FPM would like to extend thanks to all our members who contributed to our activities in 2021, whether as committee members, examiners, specialty advisers, educational supervisors, appraisers or by supporting raising awareness and advocacy events and policy projects. We truly value your participation and support.










































