N-Nitrosamines: What are they? Where they do arise in pharmaceuticals how concerned should we be?
Posted on: Tuesday 21 December 2021
Author: Andrew Teasdale
This FPM Deep Dive article has been prepared by Andrew Teasdale*.
It is provided for information and does not constitute advice or represent official FPM views or policy.
How to cite:
Teasdale, A (2021), ‘N-Nitrosamines: What are they? Where they do arise in pharmaceuticals how concerned should we be?’, FPM Deep Dive, 21 December 2021. Available at: https://www.fpm.org.uk/deep-dive/n-nitrosamines (Accessed: <date>).

Introduction
Few people within the pharmaceutical and certainly the wider population would have heard of N-Nitrosamines before July 2018. This changed almost overnight with reports of contamination of a Valsartan drug substance with a known animal carcinogen, Nitrosodimethylamine (NDMA), A highly potent carcinogen. This led to widespread withdrawal of products containing Valsartan drug substance supplied by Zhejiang Huahai. Very quickly this spread to being an issue with material supplied by other manufacturers of not only Valsartan, but other similar products in the wider Sartan class.1,2,3
Stepping back a moment, while there was little knowledge of N-Nitrosamines prior to this, it is important to realise that the risk in itself was not a new one, there being multiple sources of N-Nitrosamines, ranging from the water we drink, to the food we eat and even cosmetics we wear.
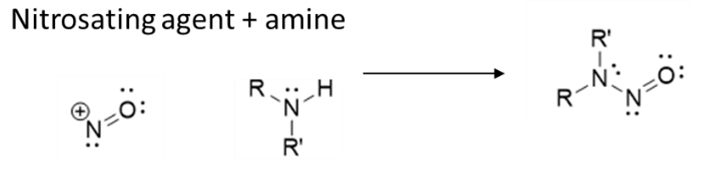
In reality, the carcinogenic properties of nitrosamines have been known for more than 50 years. Indeed, the presence of low-level nitrosamines within the human environment is ubiquitous. Sources include tobacco (smoking and chewing), rubber products, cosmetics (creams, lotions, shampoos) and food. Indeed, in terms of food specifically, Nitrosamines are present in foods such as bacon, beer, and preserved fish. They can also be formed when meats are heated to high temperatures. Nitrosamines can also be found in drinking water. Perhaps even more intriguing is that Nitrosamines are also formed endogenously in our body, formed through nitrosation of ingested amines reacting with nitrites.
At a recent FDA Webinar focused on N-Nitrosamines the reported levels of endogenous exposure were enlightening:
- It has been estimated that levels of NDMA specifically range from 100 to 2500 ug/day.
- Measurement of specific DNA adducts correlate well with this
Despite the uncertainties surrounding the accuracy of such measurements it is difficult not to reflect on such levels and pose the question:
How does the risk posed by contaminated pharmaceuticals relate to the overall exposure to N Nitrosamines?
So what happened?
In the case of Valsartan, the issue was triggered by a process change, this being made to improve the efficiency of the synthesis. Valsartan (this is true of almost all other Sartans as well), contains a tetrazole ring, to make this requires application of a specific type of chemistry, one aspect of this is the use of a reactive chemical called an azide. The process change saw the use of a more reactive, more efficient reagent, sodium azide. However, because of the higher reactivity at the end of reaction, excess reagent needs to quenched, this is done using another reagent, sodium nitrite. As well as this change the solvent system was changed and a new solvent Dimethyl formamide (DMF) used. Even chemists may say ‘so what?’ at this point.
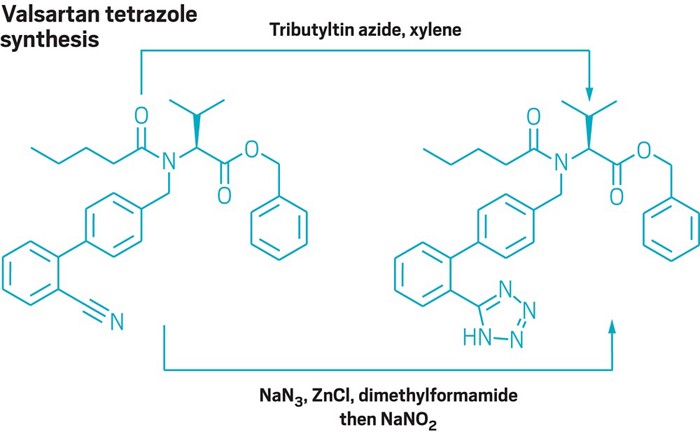
Image https://cen.acs.org/pharmaceuticals/process-chemistry/side-reaction-led-impurities-found/97/web/2019/02
Let’s examine this. So what happened? N-Nitrosamines are formed through reaction between a nitrosating agent and a secondary amine. By using sodium nitrite the revised process now has a nitrosating agent present (nitrite in water yields nitrous acid). For a reaction to occur you need a secondary amine, unfortunately DMF contains dimethylamine (DMA) as an impurity and also can degrade to DMA. Thus although entirely unwittingly the result nevertheless was almost perfect conditions to yield NDMA.
What happened next?
Of course sadly the issue of N-Nitrosamines in pharmaceuticals did not stop there, N-Nitrosamines were also seen to be present in drug products where the source could not be explained by the chemistry. Contamination was seen due to contaminated reagents, in particular contaminated recovered solvents. It did not stop there either. In 2019 another major drug Ranitidine was reported to contaminated with NDMA4, in this case by an entirely different mechanism related to the intrinsic nature of the molecule and an intermolecular reaction.5
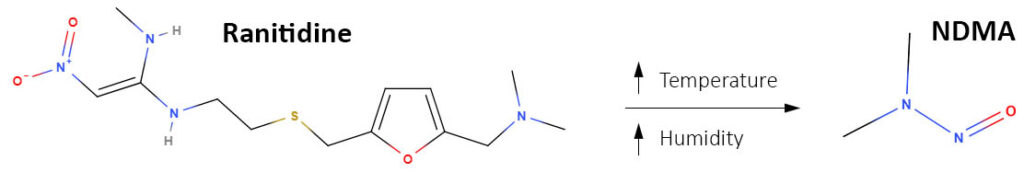
Further assessment
Further risks were reported for other products, a chronology of which is shown below. As part of this further risk were identified, specifically:
- Risks associated with the generation of N-Nitrosamines during drug product manufacture and stability. This relates typically to generation of an N-Nitrosamine through reaction of a secondary amine ‘impurity’ in the API and traces of nitrite in excipients used in formulated product. That excipients contain traces of nitrite is an established fact, levels at or around 1ppm are common. In most scenarios this is insufficient to be of real concern. There is though one critical exception to this, this being where the active is a secondary amine. In this scenario ppm levels of Nitrite are more than sufficient to form levels of N-Nitrosamines of concern, especially where limits as low as 18ng/day apply. This is a point returned later in the article.
- Risks associated with packaging, particularly lidding foils where nitrocellulose based materials are involved.

Current understanding indicates the risk to be low, a few nanograms per cavity, this is believed to be due a number of factors, these include:
- Very low volatilization rates of NDMA and NDEA from common blister lidding and the short time of applied heat during sealing.
- Convoluted pathways for vapours to migrate upstream into open blister wells
- Depletion of any volatilized nitrosamines from vapor cloud due to room air changeovers is nevertheless an identified risk.
Outlined below is an outline of further identified product risks and their timeline
Timeline: Key Events and Affected Products
https://www.fda.gov/drugs/drug-safety-and-availability/information-about-nitrosamine-impurities-medications
- NAs in ARBs (Valsartan): OGD alerted
- CDER NA Task Force activated
- FDA publishes analytical methods for NDMA and NDEA in valsartan API and DP methods for NDMA in
- ARB product recalls begin
Widening of the scope
Perhaps not surprisingly, this then led to concerted action by regulatory authorities, action requiring that the risk of N-Nitrosamines be reviewed for all drug products, literally thousands of products. Initially it was proposed that this be completed within 6 months. This proved impossible, especially when the impact of COVID-19 hit. Ultimately, the completion of the first phase of this – Step 1 – high level risk / no risk – was moved to 31 March 2021 in most major territories.
So what happened? Authorities reported a high level of compliance in terms of submission of assessments. Also encouragingly, despite the variety of causes described, 80-90% of products were reported to be of no risk. Of those where a risk was potentially identified the next phase has started, to clarify and ultimately quantify risks. Considerable effort is now focused on this, part of which is developing analytical methods capable of analysing for what in many cases would be parts per billion
So where are we now?
As highlighted, most products are not a concern. However, challenges still remain in terms of investigating those products at risk and even the basis for the investigations. Outlined below are some of the challenges
Acceptable Limits for N- Nitrosamines
Authorities have published proposed limits for common N-Nitrosamines, like NDMA. However, even for common N Nitrosamines the associated carcinogenicity is often of poor quality, certainly in terms of current requirements for such studies. This opens the debate of ‘can these data be used or is new data needed?’ This becomes even more acute when the safety profile of a novel N-Nitrosamine is considered. How therefore should a limit for an N-Nitrosamine of an actual API be determined? While the quality of studies performed historically may be questioned, it would seem reasonable to assume that they are able to discriminate between potent N-Nitrosamines and others that are less potent so it would seem extreme to simply default to a value of 18ng/day irrespective of the data already generated simply because it did not satisfy the criteria now set for carcinogenicity studies. Indeed, there is now good and increasing evidence that the potency of larger, more bulky, N-Nitrosamines is very different to small molecular weight N-Nitrosamines such as NDMA. Should they be treated the same? Many would argue no, that as data increase so does the ability to discriminate based on factors such as size, shape and other factors.
Also our understanding of the reaction has increased, recent models allow for accurate prediction of levels of N-Nitrosamines formed under process and formulation conditions. This allied with appropriate safety limits and analytical data, is starting to show that even for those products deemed a risk after the end of Step 1, they are in reality not of concern.
So what next?
Risk assessments need to continue but also crucial is development of appropriate regulatory guidance. The current guideline covering mutagenic impurities, ICH M7 does not entirely address N Nitrosamines and current specific guidance is regional and varied, both in content and also implementation. Without a common set of principles, industry will continue to grapple with the issue, putting at risk the continuity of supply of critical medicines.
How significant is the patient risk?
A final thought, and back to the issue of patient safety and the case of Ranitidine. It was assumed that levels of NDMA formed in drug product would impact on patient safety as levels seen in some products exceed permitted levels and may also form in vivo after administration.
Indeed a paper in Carcinogenesis, was published stating that “Oral intake of ranitidine increases urinary excretion of N-Nitrosodimethylamine,” This was written by researchers at Syracuse University and Stanford University. Not unexpectedly, it received widespread media coverage. However, this was recently retracted. The retraction notice states: “the FDA has found that the connection between ranitidine and NDMA that the authors reported might have resulted from issues with the instruments they used to test their samples: Recent research has identified the potential for an analytical artefact associated with the use of gas chromatography that could have contributed to the levels of N-Nitrosodimethylamine (NDMA) measured in urine samples containing ranitidine in this study. Given this artefact, the authors have informed the journal that their NDMA measurements are not reliable.”
This highlights the importance of good scientific process in all aspects of assessments surrounding N-Nitrosamines.
References
- EMA Press Release 5th July 2018, https://www.ema.europa.eu/en/news/ema-reviewing-medicines-containing-valsartan-zhejiang-huahai-following-detection-impurity-some
- FDA News Release, https://www.fda.gov/news-events/press-announcements/fda-provides-update-its-ongoing-investigation-valsartan-products-and-reports-finding-additional
- FDA News Release https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-angiotensin-ii-receptor-blocker-arb-recalls-valsartan-losartan#5cc3b83d3b22e
- https://www.fda.gov/news-events/press-announcements/statement-alerting-patients-and-health-care-professionals-ndma-found-samples-ranitidine
- Ranitidine—Investigations into the Root Cause for the Presence of N-Nitroso-N,N-dimethylamine in Ranitidine Hydrochloride Drug Substances and Associated Drug Products. Fiona J. King, Andrew D. Searle, and Michael W. Urquhart Organic Process Research & Development 2020, 24, 12, 2915-2926
*Contact the author: Andrew Teasdale, Chemical Development, Pharmaceutical Technology and Development, Operations, AstraZeneca, Macclesfield SK10 2NA, United Kingdom | Email: andrew.teasdale@astrazeneca.com







