Striving for better patient care and outcomes with our drugs — pharmaceuticals and “companion” genomic testing
Posted on: Tuesday 3 October 2023
Author: Dr John Bolodeoku

Pharmacogenomics is the study of how genes influence individuals’ responses to pharmacological treatments. Pharmacogenomics can enhance patient care by enabling treatments tailored to genetic makeup and lowering the risk of serious adverse events and non-response or poor responders. As of June 2019, there were 132 pharmacogenomic dosing guidelines for 99 drugs and pharmacogenomic information is included in 309 medication labels.
Despite increasing interest in pharmacogenomics and the potential benefits to improve patient care, implementation into clinical practice has yet to be widespread, subjecting our patients to unnecessary side effects and poor efficacy with wasted time and resources.
There are some fascinating statistics that demonstrate the impact of genetics on the efficacy of medicines. For example, approximately 6.5% of UK hospital admissions are caused by adverse drug reactions, while most prescription medicines work on only 30% to 50% of people. A significant part of this is due to genetics: almost 99% of people carry at least one genetic variation that affects their response to certain drugs, including commonly prescribed painkillers, heart disease drugs and antidepressants. By the age of 70, about 90% of people are taking at least one of these medications.
Before entering Pharmaceutical Medicine, I trained as a Chemical Pathologist, which involved the biochemical investigation of body fluids, such as blood, urine, faeces, cerebrospinal fluid, etc. Over the years in Pharmaceutical Medicine, I am one of those rare individuals who has been part of the launch of a drug in the UK (as the UK Medical Adviser at Pfizer), then a practising Consultant Chemical Pathologist who prescribed lipid-lowering agents for my patients, and later a patient prescribed the very same medicines that I supported and launched. As a patient, I am now taking two products that I was involved with from my time in Cardiovascular Medicine at Pfizer. I know the adverse reactions of being on a statin and a calcium channel blocker, and I am fortunate to be at my expected LDL-cholesterol target of <1.8 mmol/L and have well-controlled blood pressure.
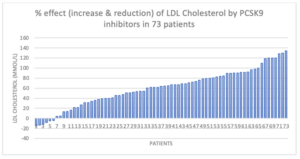
One of my patients recently asked me about interactions between statins and her medications. Then, the whole issue of drug-to-drug interactions hit me: I do not have all the appropriate drug–drug reactions at my fingertips. Additionally, whilst conducting an audit on my patients who were prescribed an enzyme inhibitor for PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9), which binds to lipoprotein receptors, only 30% of them achieved the European Atherosclerosis Society’s standard for LDL-cholesterol targets of <1.8 mmol/L, 10% discontinued treatment and 14% failed to respond to treatment (< 15% LDL reduction). The Chemical Pathologist in me questioned why we in Pharma do so much to promote efficacy but do not put the same effort into understanding and educating the clinicians on drug-to-drug reactions, adverse reactions and non-responders to the medicines we produce and market.
Measuring PCSK9 levels in the blood before and after taking medication provides some initial clues – statins have been known to increase PCSK9 expression in some cases, potentially impacting the effectiveness of inhibitors. Unfortunately, PCSK9 tests are not offered, and the current tests are far from standardised or reproducible, so this option is limited to research purposes only. Should Pharma not undertake research and develop tests as companion diagnostics? In my audit, 32% of the patients on PCSK9 inhibitors had <40% reduction of LDL-cholesterol compared to the 50 – 60%, which was the average reduction; it would have been appreciated if one could identify these patients beforehand.
If pharmacogenomic testing was available, it could make a significant difference to patient treatment and save the NHS millions of pounds. For example, about 8% of the UK population lacks the gene that allows codeine to work correctly, meaning they will not get any pain relief. Another example is the antibiotic gentamicin: about one person in every 500 is genetically predisposed to develop hearing loss if they take it.
Change is starting to happen, and some pharmacogenomic tests are available already in the NHS, for example, for a drug called abacavir, used to treat HIV, and for a drug called 5-fluorouracil, used to treat certain cancers. Nick Broughton shared an article on LinkedIn highlighting that an innovative genetic test was being used routinely in clinical practice to identify whether a critically ill baby admitted to intensive care has a gene change that could result in permanent deafness if treated with gentamicin.
Barriers to incorporating pharmacogenomic testing into clinical practice include low genomic literacy amongst physicians; drug labelling information that is difficult to interpret and/or out-of-date; clinical guidelines for pharmacogenetic testing that are sometimes discrepant (between organisations) and occasionally may be biased; and technical, as well as logistical, challenges in pharmacogenetic analysis and interpretation of results.
With my background in Chemical Pathology and Pharmaceutical Medicine, I am very keen on pharmacogenomics and the implementation of such a service that will involve doctors, pharmacists, other healthcare prescribers (both primary and secondary care), clinical geneticists, biochemists, clinical scientists involved in genetic testing (for both laboratory and potentially point of care testing); software engineers; informatics; healthcare commissioners; the public and patients. The question for Pharma is whether we should begin to carry out pharmacogenomic testing or profiling on our subjects and patients in our clinical trials.
Dr John Bolodeoku
Dr John Bolodeoku is currently a Consultant Chemical Pathologist and Pharmaceutical Physician working for the Life Sciences Sector (Pharmaceuticals, Diagnostics, Devices and Laboratories). After completing his specialist training in Chemical Pathology and Human Metabolism, he joined the Pharmaceutical Medicine Industry (1997) as a Clinical Research Physician at BIOS (Consultancy & Research) Organisation in Bagshot with its Phase I unit at Basingstoke Hospital and then went to Medical Affairs in Pfizer UK in Sandwich, Kent as Medical Adviser. He then moved to Europe to work in Medical Affairs for Yamanouchi Pharma Europe in the Netherlands as a European Medical Manager (2000). He was promoted to Director of Medical Affairs & Health Economics at Yamanouchi Pharma Europe (2021).
He went on to become the Senior Medical Director for the merged company of Yamanouchi and Fujisawa, Astellas Europe (2005), and he resigned from Yamanouchi/Astellas as Vice President for Medical Affairs and Health Economics (2009) and was awarded an Honorary Life Member Award by Astellas Europe. He started his own consulting company, JB Consulting, to broaden his reach into diagnostic pathology. JB Consulting’s pharmaceutical assignments have included Daiichi Sankyo, Lilly Diabetes, Lilly Oncology, Novartis, TEVA, MSD, UCB (Medical Adviser), NAPP (Head of Medical Affairs), Biogen Idec & Celgene (UK & Ireland Country Medical Director), Roche Ireland (Country Medical Director), ICON Solutions (Clinical Research Physician and Principal Investigator). He is an Associate of the Faculty of Pharmaceutical Medicine and has held several positions: Chairman of the Committee of Affiliates, Associates and Members (CAMM), Executive Member of the Faculty Board and Representative to the British Medical Association Medical Academic and Scientific Committee. He is a Visiting Senior Lecturer in the Centre for Pharmaceutical Medicine Research (CPMR) at King’s College London. In addition, he has been an Honorary Consultant Physician (Lipids) at North Hampshire Hospital, Basingstoke, UK, since 1999 and has been a key opinion/thought leader for the following pharmaceutical companies with lipid-lowering medication Pfizer, MSD, Novartis, Daiichi Sankyo and Sanofi.
References
- J Bolodeoku, K Morris and S Whitehead./The Association for Clinical Biochemistry and Laboratory Medicine (Publication year) Only thirty percent (30%) of patients on PCSK9 inhibitors achieve ESC/EAS LDL-Cholesterol (LDL-C) guideline target of LDL-C < 1.8 mmol/L in a specialist lipid clinic. Available at: PowerPoint Presentation (acb.org.uk)
