From Crisis to Hope: A Journey in HIV Care
Posted on: Friday 1 December 2023
Author: Dr Cynthia Wat and Flora Butler (FPM Policy Coordinator)

Introduction
As we commemorate World AIDS Day, it’s essential to reflect on the remarkable progress made in the battle against HIV/AIDS over the past few decades. Dr Cynthia Wat, whose career has been deeply intertwined with the evolution of HIV treatment, shares a firsthand account of the transformative changes witnessed on the front lines.
The 1990s: A Grim Reality
In the early 1990s, the Royal Free Hospital was a battleground against HIV/AIDS, where Cynthia was part of a multi-disciplinary team of clinicians, nurses, oncologists, and psychologists at the Ian Charleson Centre, which was set up to treat and care for patients infected with HIV. The team worked tirelessly to care for patients grappling with the classic opportunistic illnesses in immunocompromised patients, like Pneumocystis pneumonia (PCP), CMV retinitis, cryptococcal meningitis and Kaposi’s sarcoma, as well as helping patients deal with the stigma of the disease. For a night on call, there would be at least one patient, if not more, sick with PCP and having difficulty breathing. During this time, mortality rates were alarmingly high; there were 2.3 million AIDS-related deaths globally by 1998, painting a grim picture of the devastating impact of the virus.
The Era of HAART : Fuzeon and Treatment Innovation
There was an extreme sense of urgency with the rapidly increasing death toll to develop new treatments to target the virus by scientists and pharmaceutical researchers (Merck and Roche). It was clear that a combination of different targets was needed, and the advent of highly active antiretroviral therapy (HAART) was the result of this research, licensed in 1995. However, viruses can adapt rapidly and become resistant to the available treatments; this drove the accelerated licensing of Fuzeon in 2003. At Roche, Cynthia had the privilege to be part of the dedicated team focused on addressing the needs of heavily treatment-experienced, drug-resistant HIV patients.
HAART marked a pivotal moment in the fight against HIV/AIDS, saving lives and cutting the US AIDS death rate by 70%, illustrating the potential for innovation to turn the tide. Cynthia and the team worked closely with patient advocates for the design of the clinical trials and interacted with health authorities frequently, transforming the way industry, regulators, and patient advocates rapidly developed medicines together.
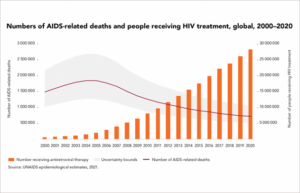
The Turn of the Century: Shifting from Crisis to Chronic Illness
Despite the advent of highly active antiretroviral therapy (HAART), it still took until 2005 for the worldwide AIDS death rates to peak until 2005 at 1.8 million. The landscape of HIV care underwent a profound transformation. Improved treatment regimens led to a striking shift from acute to chronic illness. Hospital wards, once filled to capacity with HIV patients, gradually emptied as individuals were able to control their condition effectively. Outpatient care became the norm, signalling a significant improvement in the quality of life for those living with HIV. Importantly, thanks to the global movement to improve access to prevention, care and treatment, HAART became widely available and affordable; pharmaceutical companies licensing to generic manufacturers to address treatment in lower to middle-income countries, like Sub-Saharan Africa, which remains the home to the majority of HIV infection today.
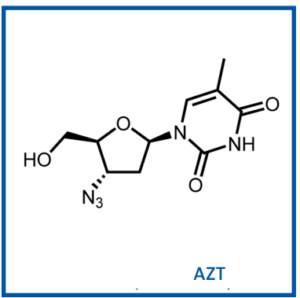
A Glimpse into the Future: Pursuing an HIV Cure
Despite the remarkable progress achieved in HIV treatment, a pressing challenge looms large – the pursuit of an HIV cure. The unmet medical need today is to raise the bar even higher, striving for a breakthrough that would eliminate the virus from the body. Many scientists and companies continue to research this challenging area, underscoring the ongoing commitment to finding a cure for HIV.
Conclusion: Hope on the Horizon
As we commemorate World AIDS Day, it’s crucial to acknowledge the global collective efforts over the last 40 years that have brought us to a point where HIV is no longer an imminent death sentence. HAART has helped to avert over 1 million deaths every year. The journey from the overwhelming crisis of the 1980s to becoming a chronic condition with patients living into their 70s-80s is a testament to the resilience of the healthcare community, the spirit of scientific innovation, and the strength of those living with HIV.
The quest for an HIV cure continues, and with each passing day, we move closer to a world where HIV is not just manageable but ultimately conquerable. As we reflect on the journey so far, let us renew our commitment to the ongoing fight against HIV/AIDS and strive for a future free from this global health threat.
Dr Cynthia Wat
Dr Cynthia Wat MBBS MRCP MFPM has more than 25 years of development experience in the pharmaceutical industry, dedicated to the development of therapies for infectious diseases & hepatitis. She is recognised for delivering smarter clinical development and known for her strong collaboration with health authorities, key opinion leaders & patient advocacy groups, transforming the way industry develops medicines. Cynthia trained at St Mary’s Hospital Medical School, Imperial College, London.
Over the course of her career, Cynthia has served in strategic leadership and managerial positions, leading global cross-functional teams, including Pegasys®, Fuzeon®, Tamiflu®, 3CLprotease inhibitor for COVID-19, siRNA/ASO, PDL1 LNA, TLR7 agonist, CpAM. Her latest role at Roche was Therapeutic Area Leader in Infectious Diseases for Early Development. She is currently an independent pharmaceutical consultant, Director of ID Pharma Consultancy Ltd, and a member of the Infectious Disease expert group at the Faculty of Pharmaceutical Medicine.
Some of Cynthia’s key highlights of her career include enabling the highest functional cure rates seen to date with a combination of NMEs for the treatment of Chronic Hepatitis B (CHB) patients via an innovative platform design; successful accelerated US & EU approvals of Fuzeon® in heavily treatment-experienced, drug-resistant HIV patients; an in-licensing deal between Roche & Dicerna Pharmaceuticals Inc. for their novel investigational GalXCTM siRNA therapeutic (Licensing Deal of the Year Scrip Award in 2020); collaborated with FDA, KOLs & industry to write & publish guidance for “Liver safety assessment in clinical trials of new agents for CHB”; pioneered the first health authority cure seminar and first paediatric CTA hearing in China.
